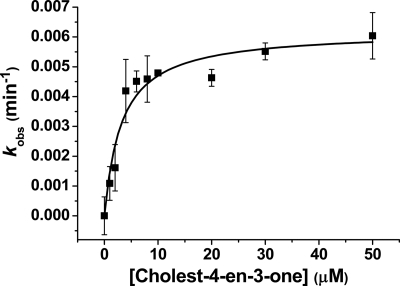
FIGURE 5.
Steady-state analysis of cholest-4-en-3-one turnover by CYP142. A CYP142 cholest-4-en-3-one-oxidizing system was reconstituted using the spinach ferredoxin reductase/ferredoxin proteins and NADPH, as described under “Experimental Procedures.” The system was effective in driving 27-hydroxylation of both cholest-4-en-3-one and cholesterol, and data for substrate-dependent NADPH oxidation versus cholest-4-en-3-one concentration were fitted using the Michaelis-Menten equation to provide parameters of kcat = 0.0062 ± 0.0004 min−1 and Km = 3.2 ± 0.8 μm.