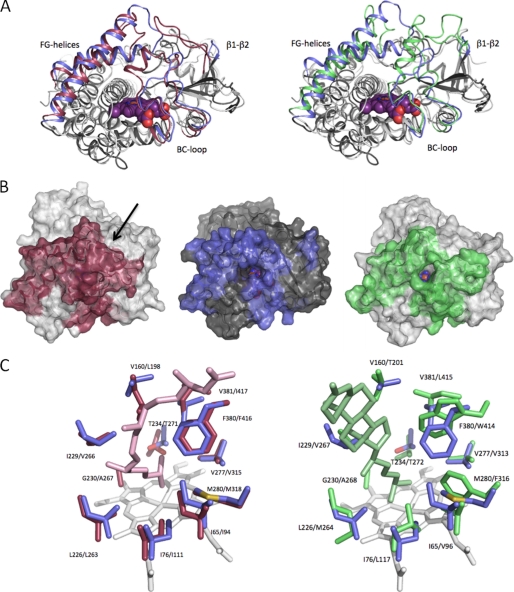
FIGURE 7.
Structural features of CYP142. A, overlay of CYP142 with CYP124 (left panel) and with CYP125 (right panel). The β1-β2-loop, BC-loop, and the FG-helices are colored blue (CYP142), red (CYP124), and green (CYP125), respectively. B, solvent-accessible surface of CYP124 (left), CYP142 (middle), and CYP125 (right), with color coding as in A. The arrow indicates the access site entry for CYP124, which is not directly visible in this orientation. In case of CYP142 and CYP125, the access channel can be readily identified by the direct view onto the heme cofactor. C, overlay of the CYP142 distal heme pocket (residues in blue) with CYP124 (left panel; in red) and CYP125 (right panel; in green). CYP124 and CYP125 substrates (phytanic acid and cholest-4-en-3-one) are also shown in the respective overlays.