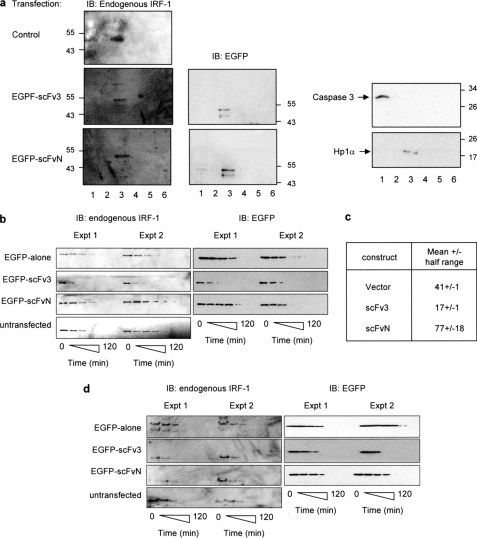
FIGURE 7.
scFv3 decreases the half-life of IRF-1. a, HeLa cells were untransfected (control) or transfected with EGFP-scFv3 or EGFP-scFvN (1.2 μg) and fractionated after 24 h. Fraction 1, cytosol; fraction 2, membrane/organelle; fraction 3, soluble nuclear proteins; fraction 4, detergent-insoluble nuclear fraction; fraction 5, cytoskeletal proteins. Fraction 6 was generated by resuspending the residual pellet in SDS sample buffer. The fractions were analyzed by SDS-PAGE/immunoblot (IB) and probed with anti-IRF-1, anti-EGFP, anti-HP1α (a nuclear localization marker), and anti-caspase 3 (a cytosolic localization marker). The data are representative of two independent experiments. b, HeLa cells were untransfected or transfected with pDEST53 (EGFP alone), EGFP-scFvN, or scFv3 (0.6 μg). Post-transfection (24 h), the cells were treated with cycloheximide (30 μg/ml) and harvested at 0, 15, 30, 45, 60, 90, and 120 min. Detergent-soluble proteins were analyzed by SDS-PAGE immunoblot developed using anti-EGFP or anti-IRF-1 monoclonal antibody. Two independent experiments are shown, and they are representative of a total of five such experiments. c, natural logarithm (ln) of (% protein remaining) was calculated for the data in b, and plotted against time (in minutes) to obtain a linear graph of the form y = mx + c (m = slope, c = y-intercept). A mean graph was drawn, and the time at y = ln(50) was calculated from the equation of the mean line. This value represents the calculated half-life (t0.5) ± half the range. d, detergent-insoluble pellet from b was solubilized in sample buffer and analyzed by SDS-PAGE/immunoblot developed using an anti-IRF-1 monoclonal antibody.