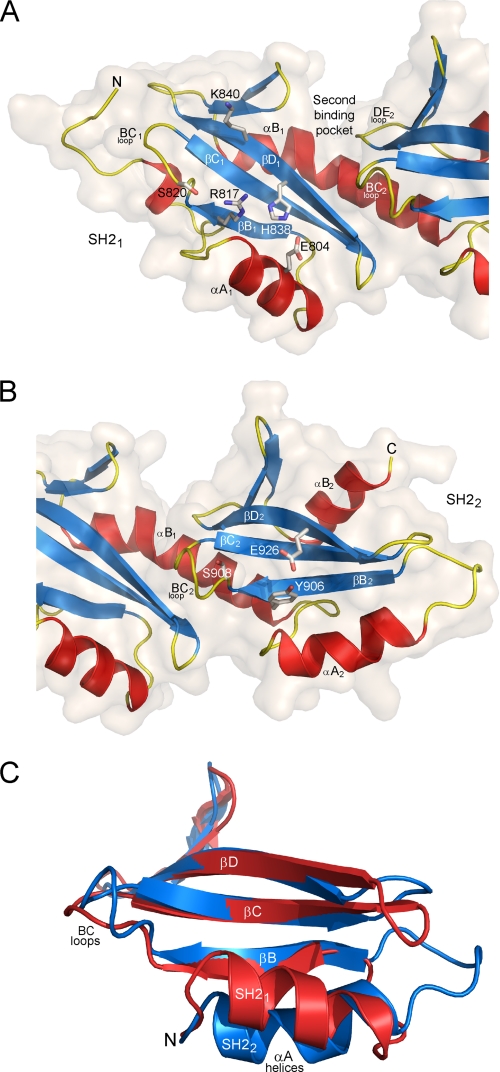
FIGURE 2.
Structural details of the SH21 and SH22 domains. A, ribbon representation of the SH21 domain. The arginine (R817) that is invariant in canonical SH2 domains is shown as sticks. The two residues (H838 and E804) forming a canonical hydrogen bond network with Arg817 are also shown as well as other residues (S820 and K840) that might be involved in recognition of an incoming phosphoresidue. The surface of the SH21 domain is shown, highlighting the groove formed between the two SH2 domains that define the second binding pocket generally observed in canonical SH2 domains. B, ribbon representation of the SH22 domain. The tyrosine (Y906) that replaces the invariant arginine of canonical SH2 domains is shown as sticks as well as the glutamate (E926) forming a hydrogen bond with the hydroxyl of this tyrosine. The serine residue (S908) from the BC2 loop that might be involved in recognition of an incoming phosphoresidue is also displayed. C, superposition of the SH21 (red) and SH22 (blue) domains, highlighting the difference of conformation between the two αA helices. For clarity, part of the N termini and the αB helices of the two domains has been removed.