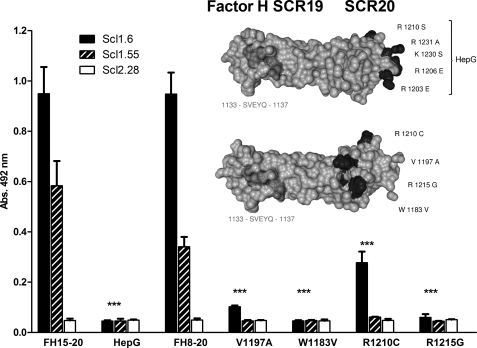
FIGURE 6.
Mutations in SCR20 and also disease-associated sequence variants affect Factor H binding to Scl1.6 and Scl1.55. Factor H SCR8–20, as well as four fragments that include aHUS-associated mutations (R1210C, V1197A, R1215G, and W1183V), and Factor H SCR15–20, which has heparin-binding residues exchanged (HepG), were attached via an immobilized monoclonal antibody (equal immobilization was verified in a parallel approach). Binding of Scl1.6, Scl1.55, and Scl2.28 applied to the fluid phase was detected using a Strep-Tag II-specific HRP-coupled antibody. Scl1.6 and Scl1.55, but not Scl2.28, bound to the unmodified fragment Factor H SCR8–20 and to Factor H SCR15–20, but the streptococcal proteins did not bind to any of the mutant proteins. Scl1.6 bound weakly to the Factor H SCR8–20 R1210C mutant construct. The mean values from triplicate wells of a representative experiment ± S.D. are shown (total of three independent experiments, ***, p < 0.001 versus no mutated proteins). The insert shows the structural model of SCR19–20 of Factor H (Protein Data Bank code: 2G7I). The positions of the five exchanged amino acids are indicated for the HepG mutant (upper panel). The disease-associated amino acids that were exchanged individually in four different aHUS cases are shown in black (lower panel). The linear Scl-binding motif is marked in dark gray.