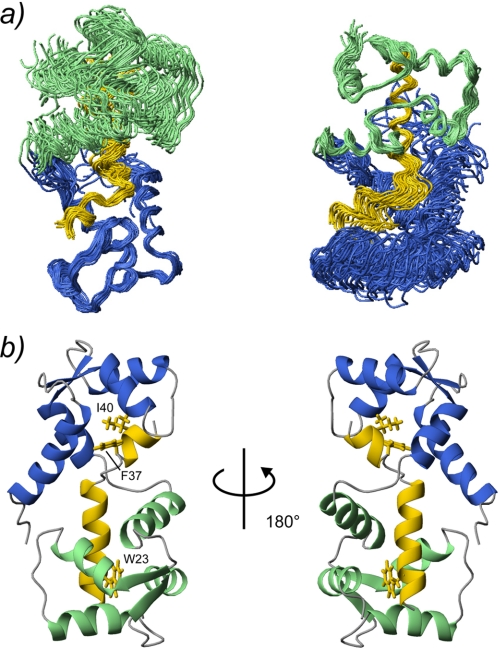
FIGURE 2.
The 25 lowest energy structures of SCaM4·BCA1 are superimposed. The N- and C-terminal lobe of SCaM4 are shown in light green and purple, respectively, whereas the BCA1 peptide is shown in yellow. Only the folded regions of the N-terminal unit (SCaM4 residues 6–74 and BCA1 peptide residues 38–43) and the C-terminal unit (SCaM4 residues 81–145 and BCA1 residues 20–33) are superimposed in the right and left panel, respectively. b, ribbon structures of the lowest energy structure of SCaM4·BCA1 at different angles. The regions are colored in the same manner as in a. The side chains of the anchor residues of the BCA1 peptide, Trp-23 and Ile-40, are shown. The side chain of Phe-37, which also has several hydrophobic contacts to SCaM4, is indicated as well.