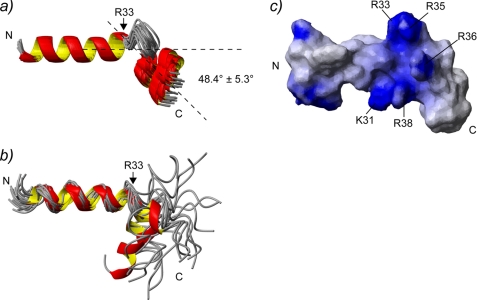
FIGURE 3.
a, the bound BCA1 peptide structures from the 25 lowest energy structures of SCaM4·BCA1. Only the first α-helical region (residues 20–33) is superimposed. The averaged angle between the first and the second α-helices for all the structures is 48.4° ± 5.3°. b, 20 BCA1 peptide structures determined in 30% trifluoroethanol are superimposed using the first α-helical region (residues 20–22). These structures were taken from Yamniuk and Vogel (20). c, the surface electrostatic properties of the BCA1 peptide. The residues that form a basic cluster in the middle of BCA1 peptide are labeled. C, C-terminal; N, N-terminal.