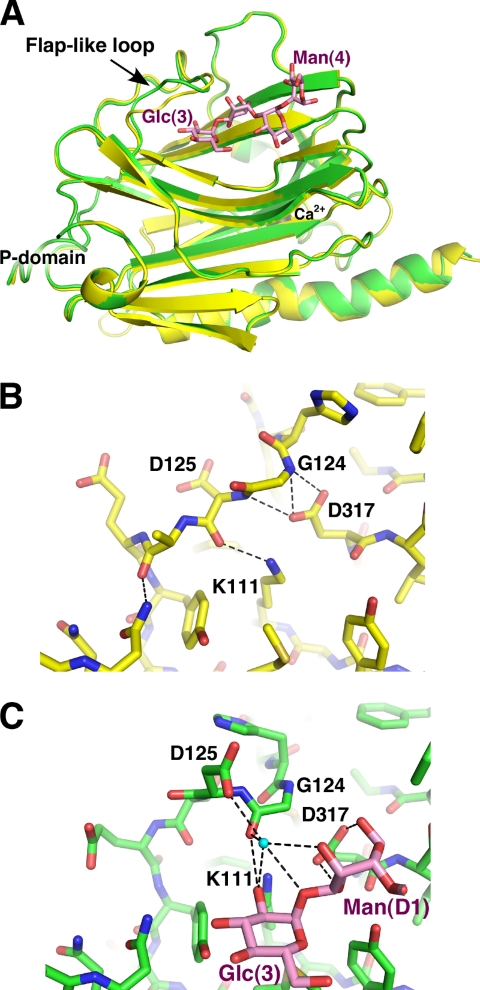
FIGURE 3.
CRT undergoes limited conformational changes upon carbohydrate binding. A, overlay of unliganded (yellow) and Glc1Man3-bound (green) CRT lectin domain structures shows differences in the large loop between strands β6 and β7. B, the conformation of the loop in unliganded CRT is stabilized by hydrogen bonds between the side chain of Asp317 and amides of Gly124 and Asp125 and between the side chain of Lys111 and carbonyl of Asp125. C, in the complex, sugar residues Glc(3) and Man(D1) engage loop residues Gly124 and Asp125 through a 60 ° rotation in the ψ backbone angle of Gly124. This rotation enables the side chain of Asp125 to participate in carbohydrate binding via an ordered water molecule (cyan sphere). The interactions of Asp317 with Gly124 are replaced by hydrogen bonds of Asp317 with Man(D1).