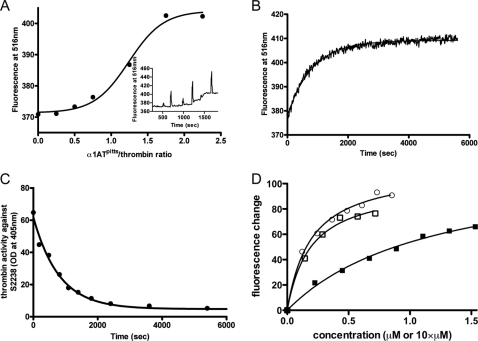
FIGURE 2.
The loss of fibrinogen γ′-peptide binding is concomitant with thrombin inhibition by serpins. A, the stoichiometry of α1ATpitts required to fully expel fluorescein-γ′ from thrombin was determined by following fluorescein-γ′-thrombin fluorescence while titrating in α1ATpitts. The inset is a time drive showing fluorescence change upon each addition (spikes reflect mixing following additions of α1ATpitts). B, time course of fluorescence change upon the addition of excess AT to a cuvette containing 50 nm fluorescein-γ′-peptide and thrombin and its corresponding fit. C, time course of loss of thrombin activity with the identical solution as in B, monitored by absorbance of chromogenic substrate S-2238 (with single exponential fit). OD, optical density. D, titration of plasma (circles), recombinant S195A (open squares), and R93E (closed squares) thrombins into solutions containing the fluorescein-γ′-peptide, with non-linear fits as lines. The concentration of plasma and S195A thrombins is μm units, whereas the concentration of R93E is 10 times higher than indicated.