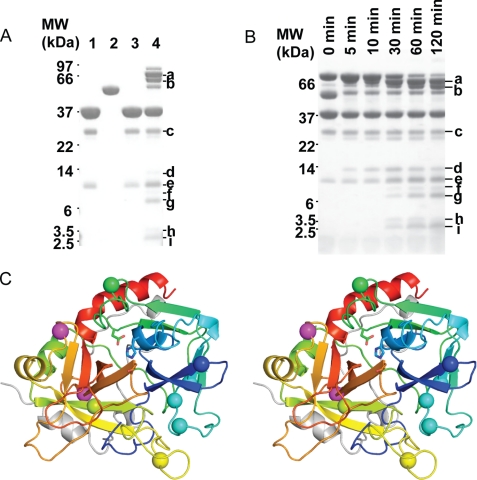
FIGURE 4.
Thrombin exosites I and II are susceptible to autolysis when in complex with AT. A, incubation of the thrombin-AT complex with excess thrombin leads to several degradation products (denoted a–i), as observed by SDS-PAGE. Lanes 1 and 2 are thrombin and AT alone without incubation; lanes 3 and 4 are thrombin alone and thrombin-AT complex (0.23 nmol of thrombin, 0.13 nmol of AT) incubated at 37 °C for 2 h. Bands a–i were subjected to N-terminal sequencing. MW, molecular weight. B, time course of thrombin digestion of the thrombin-AT complex (same ratio as in A) at 37 °C. C, stereo view of the heavy chain of thrombin in standard orientation, colored from N to C termini (blue-to-red), with identified cleavage sites indicated by balls. Magenta balls are the additional sites cleaved in the α1ATpitts-thrombin and PN1-thrombin complexes.