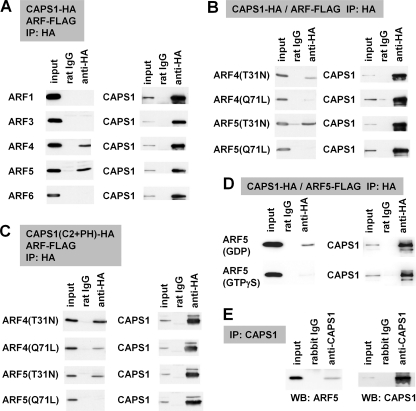
FIGURE 3.
CAPS1 interacts with GDP-locked class II ARF4/5. A–D, protein-protein interaction between CAPS1-HA constructs and ARF-FLAG constructs coexpressed in COS-7 cells was analyzed by coimmunoprecipitation (IP) with anti-HA antibody followed by immunoblotting (WB) with anti-FLAG and anti-HA antibodies. A, coimmunoprecipitation of CAPS1-HA with FLAG-tagged ARF1, ARF3, ARF4, ARF5, and ARF6; B, coimmunoprecipitation of CAPS1-HA with the GDP-locked form (T31N) and the GTP-locked form (Q71L) of ARF4 and ARF5; C, coimmunoprecipitation of the HA-tagged C2 and PH domain of CAPS1 (CAPS1(C2+PH)-HA) with the GDP-locked form (T31N) and GTP-locked form (Q71L) of ARF4-FLAG and ARF5-FLAG; D, coimmunoprecipitation of CAPS1-HA with ARF5-FLAG in the presence of GDP or GTPγS in lysis and assay buffers. E, protein-protein interaction between CAPS1 and ARF5 in mouse cerebellum in vivo. Endogenous ARF5 was coimmunoprecipitated with endogenous CAPS1 by anti-CAPS1 antibody from cerebellar lysates of P21 mice. The blots were immunostained for ARF5 (left) and CAPS1 (right).