Figure 4.
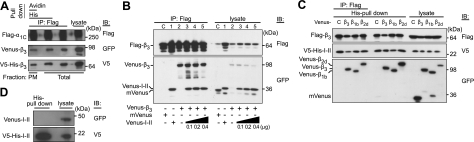
Cavβ oligomers bind to α1C at the α1C I-II linker. A) Oligomers of β3 exist in the plasma-membrane-bound α1C/α2δ/β3 channel complexes. Flag-α1C, α2δ, V5-His-β3, and Venus-β3 were coexpressed in Cos7 cells, and the plasma-membrane proteins were biotinylated. Anti-Flag IP of cell lysate isolated β3 bound to α1C. His pulldown confirmed the interaction of V5-His and Venus-β3 associated with α1C, while avidin pulldown confirmed it in the plasma-membrane fraction of α1C. B) Oligomerization of β3 is not affected by AID. Co-IP of homooligomers of Flag-β3 and Venus-β3 in the absence (lanes C, 2) or presence of increasing amounts of Venus-I-II linker containing AID (25) (0.1–0.4 μg/transfection, lanes 3–5). C) Evidence that I-II linker binds to Cavβ homo- and heterooligomers. Co-IP of Flag-β3 and V5-His-I-II linker with Venus-labeled β3, β1b, or β2d. Constructs were coexpressed in Cos7 cells. His pulldown was performed to isolate β subunits bound to AID, and the subsequent anti-Flag co-IP of the isolated β subunits was performed to check whether they were oligomers or monomers. D) Lack of oligomerization of I-II linker. His pulldown of V5-His-I-II linker and Venus-I-II linker coexpressed in Cos7 cells revealed the absence of Venus-I-II linker in the precipitate. Plasmids used for cotransfection were as following: Flag-α1C (1.2 μg), α2δ (1.0 μg), V5-His-β3 (0.5 μg), Venus-CaVβs (0.8 μg), V5-His-I-II linker (0.3 μg), and Venus-I-II linker (0.4 μg or as indicated).