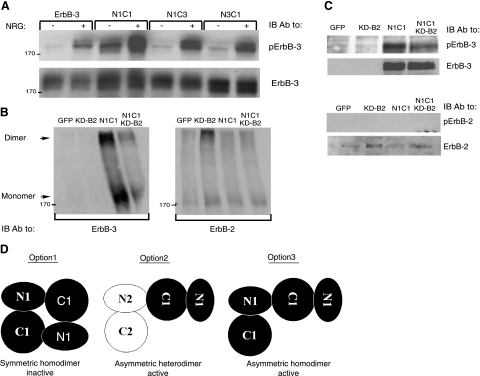
Figure 2.
The N1C1 chimera incorporates into homodimers rather than heterodimers with ErbB-2. A) Plasmids encoding wild-type ErbB-3, N1C1, N1C3, or N3C1 were transfected into CHO cells. After 48h, cells were stimulated for 5 min without or with NRG (25 ng/ml) and then washed with cold PBS. Thereafter, cells were detached, and their lysates were cleared by centrifugation, resolved by electrophoresis and then transferred onto a nitrocellulose membrane. Anti-phopsho-ErbB-3 and anti-ErbB-3 antibodies were used to monitor phosphorylation or verify expression levels, respectively. B, C) N1C1 and KD-B2 encoding plasmids (3 μg) were transfected into CHO cells, either alone or in combination (each at 1.5 μg). After 48 h, cells were washed with cold PBS, detached using a scraper, and extracted in lysis buffer with (B) or without (C) the cross-linker BS3 (2 mM). The cross-linking reaction was carried out for 20 min at 4°C and terminated by the addition of glycine (20 mM). Thereafter, lysates were cleared by centrifugation, resolved by electrophoresis, and transferred onto a nitrocellulose membrane. presence of ErbB-3 or ErbB-2 in dimers and monomers was monitored using specific antibodies. Phosphotyrosine content of both receptors was examined by using anti-phospho-ErbB-3 or anti-phospho-ErbB-2 antibodies. D) Potential dimerization modes of the N1C1 chimera are indicated. Kinase domains of the N1C1 chimera and ErbB-2 (either KD-B2 or the endogenous receptor) are represented by solid and open shapes, respectively. Option 1 denotes a symmetric inactive homodimer of two N1C1 chimeric receptors, whereas option 2 relates to heterodimerization of the chimeric N1C1 molecule together with the endogenous or the ectopic ErbB-2 molecule. Option 3 indicates an asymmetric active homodimer of two chimeric N1C1 molecules, mediated by their kinase domains.