Abstract
Background
Suitable experimental models of gallstone pancreatitis with systemic inflammation and mortality are limited. We developed a novel murine model of duct-ligation-induced acute pancreatitis associated with multiorgan dysfunction and severe mortality.
Methods
Laparotomy was done on C57/BL6 mice followed by pancreatic duct (PD) ligation, bile duct (BD) ligation without PD ligation, or sham operation.
Results
Only mice with PD ligation developed acute pancreatitis and had 100% mortality. Pulmonary compliance was significantly reduced after PD ligation but not BD ligation. Bronchoalveolar lavage fluid neutrophil count and interleukin-1β concentration, and the plasma creatinine level, were significantly elevated with PD ligation but not BD ligation. Pancreatic nuclear factor κB (p65) and activator protein 1 (c-Jun) were activated within 1 h of PD ligation.
Conclusion
PD-ligation-induced acute pancreatitis in mice is associated with systemic inflammation, acute lung injury, multiorgan dysfunction and death. The development of this novel model is an exciting and notable advance in the field.
Key Words: Mouse, Acinar cell, Nuclear factor κB, Cytokines, Acute pancreatitis, Acute lung injury, Systemic inflammatory response syndrome, Multiple-organ dysfunction syndrome
Introduction
The objective of the present communication is to report a novel murine model of duct-ligation-induced acute pancreatitis that is associated with substantial mortality. We show that death in mice with acute pancreatitis induced by pancreatic duct (PD) ligation is preceded by significant acute lung injury and organ dysfunction that potentially contribute to the high mortality in this experimental model. The pulmonary abnormalities include functional changes such as reduced compliance, morphological changes such as neutrophil infiltration, and changes in bronchoalveolar lavage (BAL) fluid such as increased neutrophils and interleukin (IL)-1β concentration. In this first report of a clinically relevant and fatal experimental model of acute pancreatitis, we present important information regarding multiple organ injury and mortality that will be invaluable for future studies using this model. With the advent of technology to genetically alter mice, such as transgenesis or in vivo gene modulation [1,2], our murine analogy of severe gallstone-induced acute pancreatitis is an important new tool to elucidate salient events in disease pathogenesis and to embark onto preliminary explorations of innovative therapeutic options.
Materials and Methods
Materials
C57/BL6, male, retired breeder mice (≥9 months of age) weighing 30–50 g were purchased from the National Cancer Institute, Frederick, Md., USA. Immunohistochemistry was performed for polymorphonuclear leukocytes (PMN), as described previously [3] (rabbit anti-myeloperoxidase, cat. No. A0398, Dako, Carpinteria, Calif., USA). Antibodies against the p65 subunit of nuclear factor κB (NF-κB), p-ERK and p-c-Jun (cat. No. 4764, 9101, 3270, Cell Signaling, Danvers, Mass., USA), TATA binding protein (cat. No. ab818, Abcam, Cambridge, Mass., USA) and β-actin (cat. No. A-5316, Sigma-Aldrich, St. Louis, Mo., USA) were used for immunoblotting.
Animal Surgery
All animal protocols were approved by the Institutional Animal Care and Use Committees. With strict aseptic precautions, midline laparotomy was performed on mice under general anesthesia induced and maintained with 2–5% isoflurane using a vaporizer and gas scavenger system. Postoperatively, the mice quickly recovered and ambulated. They were allowed free access to food and water and were given buprenorphine analgesia 0.05 mg/kg s.c. twice daily, according to the Institutional Animal Care and Use Committee protocols. The mice were monitored for physical activity, grooming routines, level of alertness, response to stimulation and other signs of stress. The mice were studied in 4 experimental groups (fig. 1):
Fig. 1.
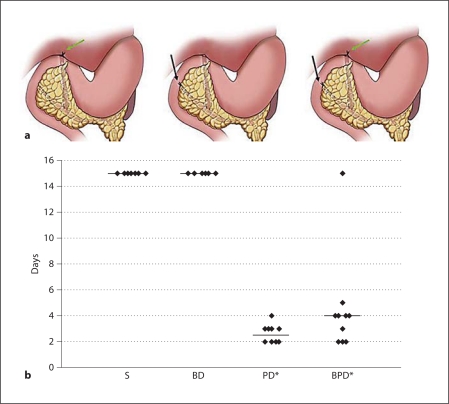
a In rodents, the BD unites with the PD after traversing posteriorly to the duodenum and pancreas. The three figures represent: left – BD ligation alone, green arrow; middle – PD ligation alone, black arrow; right – combined BD and PD ligation (BPD). b The scatter plot shows 100% mortality within 4 days (median = 2.5 days) in the PD ligation group, while sham controls (S) and BD ligation controls had no mortality during 15 days of observation. The combined BD and PD ligation group had 90% mortality at 5 days (median = 4 days) that was not different from the PD ligation group. * p < 0.05: significance for mortality versus control groups (Gehan-Wilcoxon test, n = 10 mice/group).
control groups:
group I = sham operation, i.e. the bile-pancreatic duct was dissected but not ligated;
group II = bile duct (BD) ligation alone without PD ligation;
diseased groups:
group III = PD ligation alone, the distal common bile-pancreatic duct was ligated near its junction with the duodenum;
group IV = combined BD and PD ligation (BPD), i.e. the distal common bile-pancreatic duct was ligated as in group III and the BD was ligated as in group II.
Survival Studies
For survival studies (n = 10/group), when mice showed signs of noticeable distress, they were euthanized to prevent suffering and were considered a mortality (death by itself was not an end point due to humane considerations). Mice that survived were observed for 15 days prior to elective euthanasia.
Morphological Studies
In addition to the 40 mice used for survival studies, separate mice were used to investigate events at shorter time points such as 1, 24 or 48 h. Portions of pancreas, liver, lung and kidney were harvested, fixed in neutral-buffered formaldehyde, embedded in paraffin, sectioned and stained with hematoxylin and eosin (HE). Lungs were inflated with fixative prior to excision. Selected specimens were subjected to immunohistochemistry for myeloperoxidase (MPO). All morphological and morphometric analyses were performed blinded by an independent pathologist, Dr. Meyerholz (Director, Comparative Pathology Laboratory, University of Iowa), as described previously [3].
Pulmonary Studies
Separate mice were used for pulmonary studies (n = 4–5/group). We investigated pulmonary compliance and BAL fluid characteristics in 3 experimental groups: sham, BD and PD ligation.
Pulmonary Compliance
A tracheotomy was performed on mice under general anesthesia via a midline neck incision and insertion of a 20-gauge catheter. The mice were then mounted onto a stage, monitored with ECG and temperature sensors using a Biovet C1 data acquisition system (Supertron Technologies, Newark, N.J., USA), and respiratory paralysis was induced with 0.1 mg/kg i.p. of pancuronium. The tracheal catheter was immediately connected to a small animal ventilator (Scireq Flexivent, Montreal, Canada) and ventilated at 150 breaths/min with a tidal volume of 10 ml/kg against 2–3 cm H2O positive end-expiratory pressure, under isoflurane inhalation anesthesia. Pulmonary compliance was then assessed using the forced oscillation technique [4]. Following 2 total lung capacity maneuvers to standardize volume history, pressure and flow data were collected during a series of standardized volume perturbation maneuvers. These data were used to calculate total lung compliance using the single-compartment model.
Bronchoalveolar Lavage
Through the tracheotomy tube, the lungs were filled with 1 ml of sterile saline that was then siphoned out and repeated 3 times. The BAL fluid was centrifuged at 290 g for 10 min at 4°C, and the cell pellet was resuspended in phosphate-buffered saline. After a total cell count had been performed using a hemocytometer, a smear was prepared using a Shandon Cytospin-2 cytocentrifuge (Shandon Inc., Pittsburgh, Pa., USA), and a differential cell count was performed after staining with a Hema-3 Stain Set (cat. No. 122-911, Fisher Scientific Company LLC, Kalamazoo, Mich., USA) using a light microscope (Olympus IX51, Olympus, Tokyo, Japan) under a ×60 oil immersion objective lens. The IL-1β concentration in BAL fluid was measured using a commercial ELISA kit (cat. No. DY401, R & D Systems, Minneapolis, Minn., USA).
Systemic Studies
Plasma creatinine and aspartate serum transaminase (AST) levels were measured on a Vitros Chemistry Analyzer (Vitros 350, Ortho Clinical Diagnostics, Raritan, N.J., USA). Pancreatic nuclear fractions were evaluated for activation of the nuclear transcription factors NF-κB and activator protein 1 (AP-1) with p65 and p-c-Jun immunoblots, respectively. Cytosolic fractions of the kidney and liver were evaluated for ERK activation. Plasma tumor necrosis factor (TNF)-α and IL-1β were measured with ELISA (cat. No. CMC3013, CMC0813, Invitrogen, Carlsbad, Calif., USA).
Statistical Analysis
Mortality rates were compared between groups using the Gehan-Wilcoxon test (p < 0.05). For morphometric and other studies, analysis of parametric data was made with one-way ANOVA, while nonparametric data were compared using the Kruskal-Wallis one-way ANOVA (p < 0.05), with post hoc tests for confirmation of significance.
Results
Survival Studies
In our previous study of early ligation-induced acute pancreatitis in mice, the death of a few mice between 24 and 48 h was an unexpected finding [3]. We undertook the present study to clarify whether the observed mortality was reproducible and to investigate its possible causes. Mice with PD ligation (group III) showed 100% mortality within 4 days, with a median mortality of 2.5 days (fig. 1). Mice with BPD ligation (group IV) showed near-100% mortality within 5 days and a median mortality of 4 days, that was not significantly different from group III mortality (1 mouse survived and was electively euthanized on the 15th day). Both control groups had 100% survival at 15 days when elective euthanasia was performed. In further studies, we eliminated group IV and used only 1 diseased group (PD ligation group) as there was no statistical difference in survival or morphological changes between groups III and IV.
Morphological Studies
All morphological comparisons were performed at the 48-hour time point (n ≥ 10/group).
Pancreas
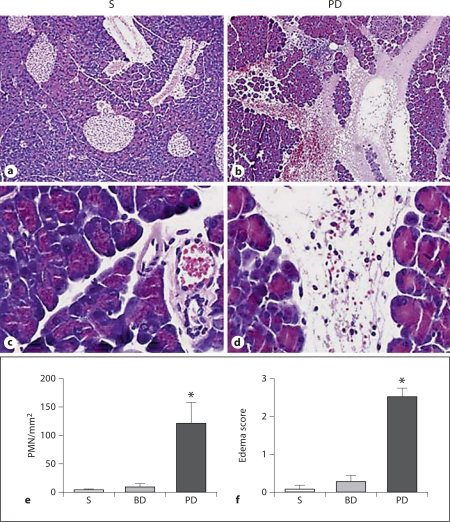
Mice in the diseased groups, but not those in the control groups, developed morphological evidence of acute pancreatitis characterized by interstitial and interacinar edema, neutrophilic infiltration, vascular congestion and hemorrhage (fig. 2). Some activated macrophages were also detected admixed with the neutrophils. Morphometric analysis of edema (range of score: 0–3) and neutrophilic infiltration showed significant increases in pancreata of PD-ligated mice but not in either control group. Mice from groups III and IV had findings not significantly different from each other (group IV data not shown).
Fig. 2.
Representative photomicrographs (HE stain) of mouse pancreas in low power (a, b) and high power (c, d) after 48 h of sham operation (S) or PD ligation. PD ligation resulted in acute pancreatitis characterized by edema, intense leukocytic infiltration, vascular congestion and hemorrhage. Graphs show pancreatic PMN count (e) and edema score (f) of the HE-stained slides. At 48 h, only the PD ligation group had evidence of acute pancreatitis while sham and BD-ligated groups did not (means ± SEM; * p < 0.05 vs. sham and BD ligation groups; n ≥ 10/group).
Lung
Morphological evaluation of HE-stained sections of lung showed increased neutrophilic infiltration of the pulmonary parenchyma of both PD and BD ligation groups (fig. 3). Morphometric analysis after immunohistochemical staining for MPO confirmed that parenchymal neutrophilic infiltration of mouse lung was increased after PD-ligation-induced acute pancreatitis and also after BD ligation alone without pancreatitis. Mild interstitial alveolar septal thickening was also noted in the PD ligation group.
Fig. 3.
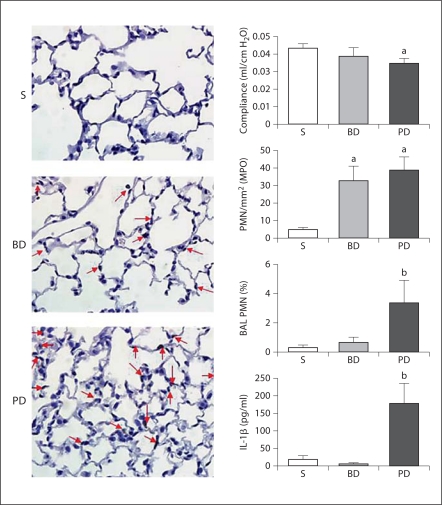
Representative photomicrographs (MPO staining) of mouse lung following 48 h of sham operation (S), BD ligation or PD ligation. Sham lungs were essentially normal morphologically. BD ligation or PD ligation resulted in vascular congestion with increased neutrophils (arrows). Graphs are described from top to bottom. Data are means ± SEM; a p < 0.05 vs. sham; b p < 0.05 vs. sham and BD ligation; ANOVA. Pulmonary compliance: after 24 h, the PD ligation group had decreased pulmonary compliance compared to sham, while with BD ligation compliance was not significantly reduced (n = 4–5/group). Morphometry of pulmonary PMN: after 48 h, the PMN count of MPO-stained lung sections in the PD ligation group was significantly higher than sham control, but was similar to the BD ligation group (MPO stain, n = 5 mice/group). BAL fluid PMN count: after 24 h, the BAL fluid showed an increased percentage of PMN after PD ligation compared to both controls (n ≥ 3/group). BAL fluid IL-1β concentration: after 48 h, ELISA of BAL fluid showed increased IL-1β concentration in the PD ligation group compared to both controls (n ≥ 3/group).
Liver
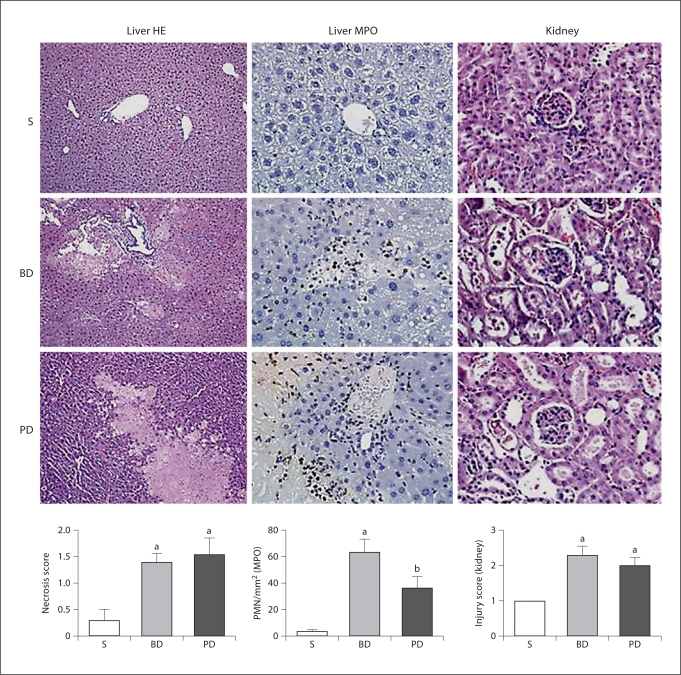
Morphological appraisal showed that the BD ligation group had small multiple foci of coagulation necrosis of hepatocytes with hemorrhage (fig. 4); the PD ligation group had moderate to large single focal coagulation necrosis, or severe diffuse necrosis, with apparent vascular thrombi. Areas of necrosis in both groups were associated with thrombi, and mild neutrophilic infiltration was confirmed with immunohistochemistry for MPO. Hepatic duct reaction to obstruction was seen as reactive proliferative epithelium with large nuclei in both groups. Morphometric analysis of liver necrosis (range of score: 0–4) and neutrophil infiltration showed that the changes in the PD and BD ligation groups were not significantly different from each other, although greater than in sham-operated controls.
Fig. 4.
Representative photomicrographs of mouse liver (HE stain and MPO stain) or kidney (HE stain) following 48 h of sham operation (S), BD ligation or PD ligation. Graphs are described from left to right. Data are means ± SEM; a p < 0.05 vs. sham; b p < 0.05 vs. sham and BD ligation; ANOVA, n ≥ 10/group. Liver necrosis score: morphometric analysis of liver necrosis shows that PD and BD groups had significant liver necrosis, compared to sham controls. Morphometry of liver PMN: morphometric analysis of MPO-stained liver sections (n = 5/group) shows that PD and BD ligation groups had significant PMN infiltration, compared to sham controls. Kidney injury score: morphometric analysis of the kidney injury shows that PD and BD ligation groups had significant renal injury, compared to sham controls.
Kidney
Sham group kidneys had very slight tubular dilation and rare sloughed cells in the lumen (fig. 4). BD and PD ligation resulted in varying extents of tubular dilation and injury with some necrotic cells sloughed or lining the tubules; intraluminal cellular debris with little pigment was also seen. Neutrophil infiltration was not evident. The renal injury score (range of score: 0–3) was higher in BD- and PD-ligated mice compared to sham, but was not significantly different from each other.
Pulmonary Function Test and BAL Fluid Analysis
As BD ligation controls had no mortality, we extended our studies to compare pulmonary compliance and BAL fluid characteristics. Pulmonary compliance was significantly reduced by 20% at 24 h in the PD ligation group, but not the BD ligation group (fig. 3). In addition, the BAL fluid neutrophil count at 24 h and the IL-1β concentration at 48 h were significantly elevated in the PD ligation group but not the BD ligation group.
Systemic Studies
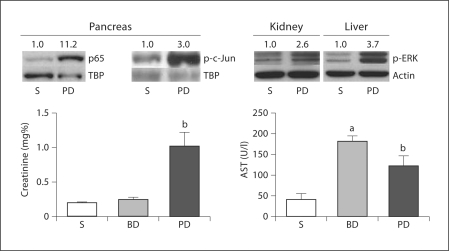
After 48 h, plasma creatinine was elevated in the PD ligation group only, while plasma AST was increased in both PD and BD ligation groups (fig. 5). The nuclear transcription factors NF-κB and AP-1 were activated in the pancreas within 1 h of PD ligation as evidenced by increased expression of p65 NF-κB subunit and p-c-Jun in nuclear fractions of pancreatic homogenates (fig. 5). We have previously shown activation of ERK mitogen-activated protein (MAP) kinase in the pancreas and lung in ligation-induced acute pancreatitis in mice [3]; here we have detected ERK MAP kinase activation within 1 h of PD ligation in the kidney and the liver (fig. 5). After 24 h of PD ligation, the mean plasma TNF-α value increased 26-fold, while the mean plasma IL-1β value increased 15-fold, over the sham control value (with BD ligation both cytokines increased only about 4-fold).
Fig. 5.
Immunoblots of pancreatic nuclear fractions show increased expression of p65 and p-c-Jun as soon as 1 h after PD ligation, compared to sham (S). Immunoblots of kidney and liver cytosolic fractions show ERK activation as soon as 1 h after PD ligation, compared to sham. Densitometry ratios normalized to nuclear loading controls (TBP = TATA binding protein) are provided above each immunoblot lane with the sham control value represented as 1. Graphs show plasma creatinine and AST concentrations after 48 h in sham, BD ligation and PD ligation groups. Data are means ± SEM; a p < 0.05 vs. sham; b p < 0.05 vs. sham and BD ligation; ANOVA; n ≥ 10/group.
Discussion
We have established a new experimental model of severe gallstone-induced acute pancreatitis that is characterized by systemic inflammation, multiorgan dysfunction and substantial mortality. In addition to a high mortality rate, we have shown that PD-ligation-induced acute pancreatitis in mice is associated with significant pulmonary inflammation and dysfunction. Compared to controls, mice with acute pancreatitis have increased bronchoalveolar inflammation, as evidenced by increased BAL IL-1β concentration and neutrophil count, and they also exhibit reduced pulmonary compliance. As the hepatic and renal morphological changes are not significantly different between BD- and PD-ligated mice at 48 h, and as median mortality is 2.5 days with PD ligation, it is reasonable to suggest that acute lung injury is most likely the major contributing factor to mortality from acute pancreatitis in this experimental model. However, it is possible that the liver and kidney also make significant contributions to expediting death from pancreatitis-induced acute lung injury in this model, and it will be interesting to conduct additional experiments to investigate these phenomena. As acute lung injury is the major determinant of morbidity and mortality in human acute pancreatitis of any cause [5], our novel model of duct-ligation-induced acute pancreatitis in mice is of particular clinical relevance. Furthermore, the analogy of PD ligation to gallstone obstruction of the PD is of greater clinical relevance in comparison to several existing experimental models of acute pancreatitis such as induction by cerulein, choline-deficient ethionine-supplemented (CDE) diet or arginine [6,7,8,9]. Finally, in vivo gene modulation techniques and the increasing availability of transgenic mice are excellent examples of the applicability of this new experimental model to investigate the pathogenesis or treatment of pancreatic inflammation, systemic inflammation, acute lung injury and multiorgan dysfunction – that lead to death in acute pancreatitis.
In a recent pilot study, we observed that 48 h of PD ligation in mice is associated with acute lung injury, stress kinase activation in the pancreas and lung, and some early mortality [3]. However, it was unclear whether the mortality was incidental, was due to the disease or due to anesthesia. Therefore, we performed the present study to ascertain whether the mortality is a consistent finding and if it is a consequence of acute pancreatitis rather than a complication accompanying anesthesia. Also, we introduced the BD ligation control to exclude hepatic causes as the primary determinant of mortality in this model. As mice with PD ligation had 100% mortality but sham-operated and BD-ligated controls had 100% survival, it is clear that the substantial mortality is an outcome of acute pancreatitis rather than of biliary obstruction or complications of the surgical procedure. In addition, we have extended our characterization of the model to include the liver and kidney in the current study.
BD ligation alone also shows morphological evidence of pulmonary neutrophilia (fig. 3) but is not associated with mortality (fig. 1). Therefore, we compared pulmonary function and BAL fluid characteristics to elucidate differences between the PD ligation group that had 100% mortality versus the BD ligation control group that had 100% survival. As expected, only the PD-ligated group – but not the BD-ligated group – is associated with functional changes of significantly reduced lung compliance and abnormal inflammatory parameters in the BAL fluid (increased neutrophil count and IL-1β concentration). We conclude that the acute lung injury manifest in mice with acute pancreatitis is of a severer form than that morphologically seen in mice with hepatic obstruction alone. It is reasonable to suggest that the functional and inflammatory manifestations observed in the lungs of mice with acute pancreatitis could contribute to the severe mortality seen in this group in contrast to the 100% survival seen in mice with BD ligation alone. On the other hand, it is both possible and probable that the effects of systemic inflammatory response syndrome and multiorgan dysfunction syndrome may play contributory, but essential, roles in propagating and expediting mortality after PD ligation in this model. For instance, as the plasma creatinine levels are elevated at 48 h after PD ligation but not after BD ligation (fig. 5), the absence of inflammatory cell infiltration of the renal parenchyma and the lack of difference in renal morphological changes in pancreatitic mice compared to BD ligation controls (fig. 4) do not indicate the absence of renal involvement in acute pancreatitis. Similarly, although plasma AST levels at 48 h are high in both PD and BD ligation groups, it is possible that these levels will remain high only in the pancreatitic mice until death while they may return to the baseline level in mice with BD ligation, as they did not die during the 2-week period of observation in the present study. There is evidence in other experimental models of acute pancreatitis that hepatic inflammation secondary to acute pancreatitis is a major source of systemic release of proinflammatory mediators [10,11]. Furthermore, it would be worthwhile to investigate cardiovascular and cerebral morphology and function in this experimental model to complete the evaluation of multiorgan dysfunction.
NF-κB and AP-1 are key nuclear transcription factors that orchestrate the production of proinflammatory mediators and are implicated in the early stages of acute pancreatitis pathogenesis and progression to systemic inflammation [1,5,12,13,14,15,16,17]. MAP kinases are upstream activators of NF-κB and AP-1. In the present study, we have shown that NF-κB (p65) and AP-1 (c-Jun) are activated in the pancreas within 1 h of PD ligation. We have previously shown MAP kinase activation in the pancreas and the lung of mice after PD ligation, and in the present study we have shown activation of the ERK MAP kinase in the liver and kidney within 1 h of PD ligation. We have also observed a trend towards increased plasma levels of IL-1β and TNF-α after 24 h of PD ligation. Activation of nuclear transcription factors and MAP kinases, increased circulating cytokine levels, elevated IL-1β in the BAL fluid, morphological evidence of significant organ injury in the lung and the liver, and increased plasma creatinine levels are clear evidence for the development of systemic inflammatory response syndrome and multiorgan dysfunction syndrome in this experimental model.
Compared to existing models of acute lung injury in acute pancreatitis, our model of PD ligation more closely resembles the human condition where gallstone impaction of the ampulla of Vater causes acute pancreatitis. Some of the existing models (e.g. cerulein-induced acute edematous pancreatitis) are not usually associated with acute lung injury, systemic inflammation, multiorgan dysfunction or mortality [6,18]. Furthermore, cerulein administration is not known to cause acute pancreatitis in humans. Existing models associated with systemic inflammation, multiorgan dysfunction and mortality (e.g. CDE-diet-induced acute pancreatitis, retrograde duct injection-induced acute pancreatitis) differ in that they do not closely mimic human etiologies of the disease [6,7,8,9]. For instance, the CDE diet is not known to cause acute pancreatitis in humans, and acute lung injury can occur with a CDE diet independently of acute pancreatitis [7]. The retrograde duct injection model and its variations are noted for the mechanical trauma to the pancreatic ductal system that occurs which may not replicate the early stages of human gallstone pancreatitis [6,8]. Finally, even 6 months of PD ligation in rats is not associated with mortality [19]. Therefore, our new model of duct-ligation-induced acute pancreatitis in mice is a unique opportunity to initiate detailed investigations into salient aspects of the early stages of the pathogenesis of acute pancreatitis and the associated mortality. As the initial treatment of acute pancreatitis is currently nonsurgical, new targets for medical treatment need to be identified [20].
In summary, we have shown that PD-ligation-induced acute pancreatitis in mice is associated with systemic inflammation, multiorgan dysfunction and death. The development of this novel murine experimental analog of severe gallstone-induced acute pancreatitis is an exciting and notable advance in the field.
Acknowledgements
This material is based upon work supported in part by the following research awards (to I.S.): (a) VA Merit Review Award, Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Biomedical Laboratory Research and Development), Washington, D.C., (b) Grant R01 DK-071731, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md., USA, and (c) American Recovery and Reinvestment Act of 2009 – Supplemental Award to NIH R01 DK-071731.
References
- 1.Rakonczay Z, Jr, Hegyi P, Takacs T, McCarroll J, Saluja AK. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259–267. doi: 10.1136/gut.2007.124115. [DOI] [PubMed] [Google Scholar]
- 2.Engelhardt JF. AAV hits the genomic bull's-eye. Nat Biotechnol. 2006;24:949–950. doi: 10.1038/nbt0806-949. [DOI] [PubMed] [Google Scholar]
- 3.Meyerholz DK, Williard DE, Grittmann AM, Samuel I. Murine pancreatic duct ligation induces stress kinase activation, acute pancreatitis, and acute lung injury. Am J Surg. 2008;196:675–682. doi: 10.1016/j.amjsurg.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Traylor ZP, Yu EN, Davis IC. Respiratory syncytial virus induces airway insensitivity to beta-agonists in BALB/c mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L437–L445. doi: 10.1152/ajplung.00363.2009. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee AK, Galloway SW, Kingsnorth AN. Experimental models of acute pancreatitis. Br J Surg. 1994;81:1096–1103. doi: 10.1002/bjs.1800810805. [DOI] [PubMed] [Google Scholar]
- 7.Chan YC, Leung PS. Acute pancreatitis: animal models and recent advances in basic research. Pancreas. 2007;34:1–14. doi: 10.1097/01.mpa.0000246658.38375.04. [DOI] [PubMed] [Google Scholar]
- 8.Hue SK, Cuthbertson C, Christophi C. Review of experimental animal models of acute pancreatitis. HPB (Oxford) 2006;8:264–286. doi: 10.1080/13651820500467358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mole DJ, McFerran NV, Diamond T. Differential preservation of lipopolysaccharide-induced chemokine/cytokine expression during experimental pancreatitis-associated organ failure in rats shows a regulatory expressed phenotype. Pancreatology. 2008;8:478–487. doi: 10.1159/000151775. [DOI] [PubMed] [Google Scholar]
- 10.Gukovskaya AS, Murr, Denbam EW. Liver injury during acute pancreatitis: the role of pancreatitis-associated ascitic fluid (PAAF), p38-MAPK, and caspase-3 in inducing hepatocyte apoptosis – discussion. J Gastrointest Surg. 2003;7:208. doi: 10.1016/s1091-255x(02)00134-8. [DOI] [PubMed] [Google Scholar]
- 11.Murr MM, Yang J, Fier A, Kaylor P, Mastorides S, Norman JG. Pancreatic elastase induces liver injury by activating cytokine production within Kupffer cells via nuclear factor-kappa B. J Gastrointest Surg. 2002;6:474–480. doi: 10.1016/s1091-255x(01)00071-3. [DOI] [PubMed] [Google Scholar]
- 12.Twait E, Williard DE, Samuel I. Dominant negative p38 MAP kinase expression inhibits NF-kappaB activation in AR42J cells. Pancreatology. 2010;10:119–128. doi: 10.1159/000290656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuel I, Tephly L, Williard DE, Carter AB. Enteral exclusion increases map kinase activation and cytokine production in a model of gallstone pancreatitis. Pancreatology. 2008;8:6–14. doi: 10.1159/000114850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sempere L, Martinez J, de ME, Lozano B, Sanchez-Paya J, Jover R, Perez-Mateo M. Obesity and fat distribution imply a greater systemic inflammatory response and a worse prognosis in acute pancreatitis. Pancreatology. 2008;8:257–264. doi: 10.1159/000134273. [DOI] [PubMed] [Google Scholar]
- 15.Szabo G, Mandrekar P, Oak S, Mayerle J. Effect of ethanol on inflammatory responses: implications for pancreatitis. Pancreatology. 2007;7:115–123. doi: 10.1159/000104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Criddle DN, McLaughlin E, Murphy JA, Petersen OH, Sutton R. The pancreas misled: signals to pancreatitis. Pancreatology. 2007;7:436–446. doi: 10.1159/000108960. [DOI] [PubMed] [Google Scholar]
- 17.Hofner P, Balog A, Gyulai Z, Farkas G, Rakonczay Z, Takacs T, Mandi Y. Polymorphism in the IL-8 gene, but not in the TLR4 gene, increases the severity of acute pancreatitis. Pancreatology. 2006;6:542–548. doi: 10.1159/000097363. [DOI] [PubMed] [Google Scholar]
- 18.Adler G, Hupp T, Kern HF. Course and spontaneous regression of acute pancreatitis in the rat. Arch A Pathol Anat Histol. 1979;382:31–47. doi: 10.1007/BF01102739. [DOI] [PubMed] [Google Scholar]
- 19.Boquist L, Edstrom C. Ultrastructure of pancreatic acinar and islet parenchyma in rats at various intervals after duct ligation. Virchows Arch A Pathol Pathol Anat. 1970;349:69–79. doi: 10.1007/BF00548522. [DOI] [PubMed] [Google Scholar]
- 20.Lankisch PG. Treatment of acute pancreatitis: an attempted historical review. Pancreatology. 2010;10:134–141. doi: 10.1159/000255465. [DOI] [PubMed] [Google Scholar]