Abstract
Anthropogenic factors, including climate warming, are increasing the incidence and prevalence of infectious diseases worldwide. Infectious diseases caused by pathogenic parasites can have severe impacts on host survival, thereby altering the selection regime and inducing evolutionary responses in their hosts. Knowledge about such evolutionary consequences in natural populations is critical to mitigate potential ecological and economic effects. However, studies on pathogen-induced trait changes are scarce and the pace of evolutionary change is largely unknown, particularly in vertebrates. Here, we use a time series from long-term monitoring of perch to estimate temporal trends in the maturation schedule before and after a severe pathogen outbreak. We show that the disease induced a phenotypic change from a previously increasing to a decreasing size at maturation, the most important life-history transition in animals. Evolutionary rates imposed by the pathogen were high and comparable to those reported for populations exposed to intense human harvesting. Pathogens thus represent highly potent drivers of adaptive phenotypic evolution in vertebrates.
Keywords: disease, life-history evolution, maturation reaction norm, pathogen, perch, size at maturation
1. Introduction
Anthropogenic factors, including climate warming, are increasing the incidence and prevalence of infectious diseases in many ecosystems worldwide [1,2]. Although knowledge about evolutionary changes induced by infectious diseases is critical to mitigate ecological and economic effects [3], rates of disease-induced adaptive evolution are largely unknown [2]. Analogous to predation or harvesting, infectious diseases caused by pathogenic parasites can have severe impacts on the survival and future reproductive success of individuals in a population [4,5]. Several empirical studies suggest that pathogens can cause evolutionary responses in life-history traits [6–9]. However, disentangling genetic and plastic effects is difficult without long-term, multi-generation studies. Hence, evidence of disease-induced evolution in reproductive investment or size at maturation, the most important life-history transition in animals, is scarce in general [10,11] and still lacking in vertebrates.
Theoretical models on the life-history evolution of parasitized hosts predict that parasitism favours increased energy allocation into host reproduction, suggesting that infected hosts will shorten their pre-reproductive periods [12,13]. These models are in line with general life-history theory, which predicts that natural selection leads to a maximization of overall lifetime reproductive success and thus that high adult mortality should select for individuals that mature earlier and invest more energy in current reproduction. Size-selective mortality has been shown to induce rapid evolutionary changes in age and size at maturation in natural populations (for instance when arising from natural predation [14,15] or human harvesting [16,17]). Harvest selection has been put forward as a particularly strong agent of phenotypic change [18]. Studies of fisheries-induced life-history evolution [16,19] have repeatedly and successfully applied the probabilistic maturation reaction norm approach [20–22], which is a tool for disentangling phenotypically plastic and evolutionary changes in the maturation schedule. It overcomes the confounding effects of growth and mortality (which depend on environmental factors) by estimating maturation probabilities that are conditional on individuals having reached a certain age and size. Therefore, a change in the probabilistic maturation reaction norm is strongly indicative of an evolutionary change in the maturation process itself [17,20]. This approach has never before been applied in the context of infectious diseases.
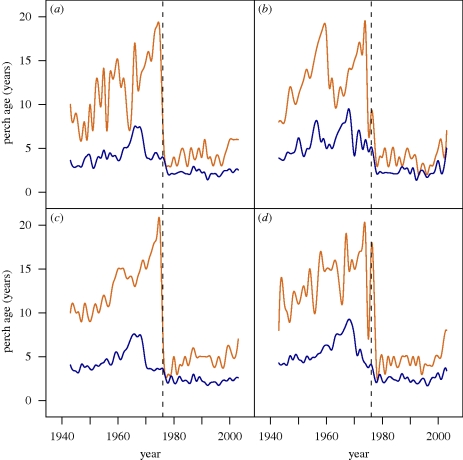
Here, we use a time series from long-term scientific monitoring of perch (Perca fluviatilis) in the lake of Windermere (UK) to estimate temporal trends in size at maturation before and after the outbreak of a disease that severely impacted the perch populations in the two basins of the lake. The two populations are independent, according to capture–mark–recapture and genetic data [23,24]. In 1976, a perch-specific pathogen induced a 98 to 99 per cent adult mortality during that year, with much higher pathogen prevalence among large, mature individuals compared with small, immature ones [25,26]. Although the pathogen could not be identified, epidermal lesions with succession of fungal colonization were apparent on the perch [25,27] and diseased individuals are still occasionally observed during the yearly samplings, although not on the scale of the 1976 outbreak. The population age structure of the perch remained severely truncated for many years after 1976, indicating that the pathogen persisted (figure 1). The disease considerably reduced the duration of the mature stage and led to a phenotypic change to smaller, slower-growing perch [26]. Edeline et al. [26] previously examined how the perch pathogen changed food-web structure through shifts in perch body size. Here, we focus on perch reproductive schedule specifically to test predictions from evolutionary epidemiological and general life-history theory. We specifically hypothesized that the pathogen infection selected for an increased investment into earlier reproduction and thus a smaller size at maturation.
Figure 1.
Demographic change in perch caused by the pathogen infection. Time series are shown of mean (blue) and maximum (brown) age for (a) males in north basin, (b) females in north basin, (c) males in south basin and (d) females in south basin. The dashed vertical line indicates the 1976 cohort, the year of the pathogen outbreak.
2. Material and methods
(a). Sampling
The scientific sampling of perch in Windermere started in 1943, and continues today with very little change in gear type and fishing methods. Perch trapping takes place for six weeks on the spawning grounds with standard traps that are unselective for perch of 90–300 mm total length [28]. Each individual fish is measured for total length and sexed by internal examination, and the left opercular bone is removed for age determination. Owing to the trapping being located on the spawning grounds, the catch contains disproportionately high numbers of mature compared with immature individuals, as well as male compared with female individuals.
(b). Estimating the probabilistic maturation reaction norm
The method we use is based on estimating proportions of mature individuals as functions of age and size, referred to as ‘maturity ogives’ [21,22]. Our procedure involves four estimation steps: (i) estimation of the probabilities of being mature (the maturity ogives); (ii) estimation of mean annual growth rates; (iii) estimation of the probability of maturing and calculation of the reaction norm mid-points; and (iv) estimation of confidence intervals around the maturation reaction norm mid-points.
The probabilities of being mature at a given age (a) and size (s), modelled as total body length of the fish, were estimated by fitting logistic regression models to the observations. Age and size were modelled as variables and cohort (c) as a factor. Here, a cohort designates all members of the population born within a particular year. The total number of cohorts in the dataset varied between 66 and 70, depending on sex and basin (maturation reaction norms could not be estimated for all of these cohorts; see below). We tested a set of a priori candidate models for the logistic regression and selected the best model for each sex based on AIC values (see electronic supplementary material, table S1). Tested explanatory variables, besides age and size, were perch and pike (Esox lucius) density in the previous year, perch condition (relative condition factor [29]) and three different temperature measures (winter, spring and summer), which were calculated as mean values during an individual's lifetime. In males, the model with most support in both basins included cohort, age, size and the cohort–size interaction term: logit (o) = β0 + β1 · s + β2 · a + β3,c + β4,c · s, whereas in females it also included the cohort–age interaction: logit (o) = β0 + β1 · s + β2 · a + β3,c + β 4,c · s + β5,c · a, with each β representing a regression coefficient. Mean annual size increments (Δs) were estimated as the difference in mean body length between a cohort at any given age (a) and the same cohort at the previous age (a − 1). Average cohort-specific size was directly calculated for females age 2 and 3 and for males age 2. The average size of males age 1 was calculated from cohort-specific von Bertalanffy growth curves fit to all age-classes of the respective cohort according to LvonB (a) = L∞ (1 − e−k·a), where k is the growth rate parameter and L∞ is the asymptotic length. The probability of maturing at a given age and size (m(a,s)) was derived from the probability of being mature at that age and size (ogive o(a,s)) and the probability of being mature at the previous age and size (ogive o(a − 1, s − Δs(a))), which was based on the mean annual growth increment (Δs): m(a,s) = o(a,s) − o(a − 1, s − Δs(a))/1 − o(a − 1, s − Δs(a)). The probability of maturing is thus calculated as the frequency of individuals that have matured over the frequency of individuals that could have matured. The reaction norm midpoints (i.e. age-specific lengths at which the fish reach a 50% probability of maturing) were then estimated by fitting a logistic regression model to these estimates, independently for each cohort. Since the use of this equation may occasionally lead to unrealistic estimates for the probability of maturing in cases where the data are insufficient [21], we restricted our age- and cohort-specific logistic regression to cases that consisted of at least five data points, had probability estimates below and above the midpoint (0.5), and showed increasing probabilities of maturing with size. Confidence intervals for the reaction norm parameters were obtained through bootstrap techniques [30]. Bootstrapped samples were constructed by randomly choosing individuals with replacement from each cohort (same number of observations as in the original sample). The resampling was repeated 1000 times, and the confidence intervals were estimated using the 2.5 and 97.5 percentiles as lower and upper limits, respectively. All computations were performed using the data analysis system R [31].
(c). Breakpoint analysis and evolutionary rates
To analyse whether the trend in the maturation reaction norm changed during the time series (and, if so, for which cohort), we performed a breakpoint analysis using the R add-on package segmented [32]. The approach assumes that the relationship between response and explanatory variable is piecewise linear; that is, two or more linear relationships that are connected at unknown values, referred to as breakpoints. The parameters are estimated by a segmented standard linear regression using an iterative fitting process. To set the initial value to the fitting process, we inspected the fitted values of locally weighted non-parametric models (loess, smoothing parameter 0.5) and visually inferred a candidate threshold as the starting value. Deviating initial values resulted in identical breakpoint estimates. The same non-parametric bootstrapping as described for the reaction norms was applied to obtain confidence intervals on the estimated breakpoint. Rates of phenotypic change for the two periods were calculated as ‘Darwin’ numerators [18,33] as d = ln (x1) − ln (x2), where x is the mean trait value at the beginning (x1) and end (x2) of the respective period.
(d). Method validation using synthetic data
To test the robustness of the estimation procedure and to rule out potential errors owing to biases or confounding effects between variables, we constructed an array of synthetic datasets using a maturation process with (i) constant or time-variable growth; (ii) constant, linearly increasing or decreasing, or piecewise linear changes in maturation trends over time; and (iii) different degrees of bias between mature and immature fish (similar to the bias in the catch data). The synthetic datasets were constructed by assuming that the individual fish follow deterministic von Bertalanffy growth. Each cohort contained the same number of individuals for a given age as in the catch data, and each individual was assigned a random set of von Bertalanffy growth parameters (L∞ and k) drawn from a normal distribution centred at the mean of the cohort. Maturation was assumed to be a stochastic process and the modelled reaction norm changed with both time and age. Each individual with a deterministic length longer than the individual random reaction norm was considered mature. Using these simulated data, we tested our method by estimating the maturation reaction norm and comparing the estimates with the known theoretical input (electronic supplementary material, figure S1). The tests showed (i) that the bias in the catch towards mature individuals might eliminate a few cohorts from the estimations, (ii) that the breakpoint analysis depicts changing trends in the reaction norm correctly and (iii) that the estimated maturation reaction norm is independent of the temporal trend in cohort-specific growth. The method thus successfully estimates the reaction norm midpoints and the breakpoints. Based on these tests, we conclude that our data are sufficient for estimating probabilistic maturation reaction norms using this method.
3. Results
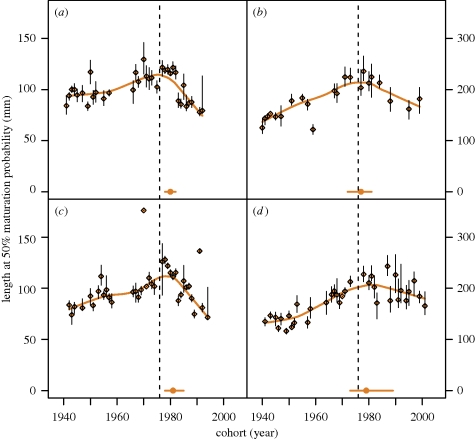
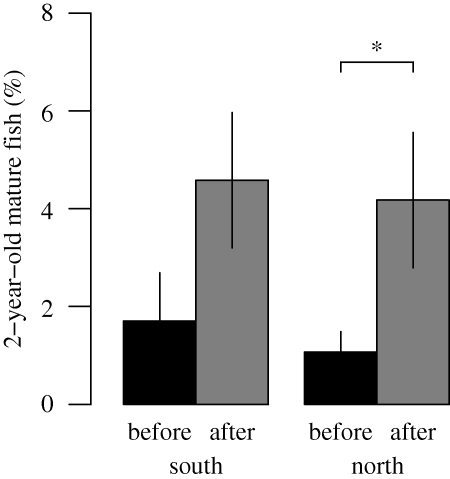
We were able to estimate temporal trends in size at maturation for 3-year-old females and 2-year-old males, corresponding to the size classes at which most of the fish mature. These trends of the maturation reaction norm strongly suggest that the disease outbreak led to a consistent evolutionary change in this life-history trait in both sexes and basins (figure 2). The estimated reaction norm midpoints (i.e. the age-specific body length where the fish reaches a 50 per cent probability of maturing) increase until a few years after the disease outbreak, and continuously decrease thereafter. For instance, the body length at which male perch reach a 50 per cent probability of maturing has decreased from 126 mm in 1977 to 72 mm in 1994 in the south basin and from 122 mm in 1977 to 79 mm in 1992 in the north basin (figure 2). Since most individuals mature within a single age class, and owing to the truncated age structure of the population, we were unable to estimate reaction norm midpoints for other ages. We therefore calculated the proportion of first-time spawners in 2-year-old females before and after the outbreak (catch numbers of first-time spawners in 1-year-old males were insufficient). This proportion increased after 1976 in both basins (figure 3), suggesting that the maturation schedule also shifted towards younger fish in response to the pathogen. A Welch t-test showed that this increase was not significant in the south basin (p = 0.122), but was significant in the north basin (p = 0.045) at the 0.05 level.
Figure 2.
Temporal trends in maturation reaction norm midpoints. Estimated lengths at 50 per cent maturation probability for each cohort and their 95 per cent confidence intervals are shown for (a) 2-year-old males in north basin, (b) 3-year-old females in north basin, (c) 2-year-old males in south basin and (d) 3-year-old females in south basin. Lines (brown) were fitted using loess smoother with a smoothing parameter (span) of 0.5 to illustrate the temporal trends in the maturation reaction norm. The estimated breakpoints at which the trend shifts from positive to negative are indicated at the bottom of each panel (brown circles with 95% confidence intervals). The dashed vertical line indicates the 1976 cohort, the year of the pathogen outbreak.
Figure 3.
Percentages of 2-year-old mature females before and after the pathogen outbreak. The mean values for the populations in the south and north basins (error bars indicate 1 s.e.m.) were compared using the Welch t-test, since homogeneity of variance was violated. The increase in first-time spawners after the disease outbreak was not significant in the south (p = 0.122), but significant in the north basin (p = 0.045).
The breakpoint analyses on the temporal trends in size at maturation identified the cohort where the trend changed from being positive to being negative to be between 1977 and 1981, depending on sex and basin, thus representing a 1–5 year lag in the response to the pathogen outbreak. Fitting two linear regressions continuously to the reaction norm midpoints before and after the disease suggests that both trends are significantly different from a zero trend (see electronic supplementary material, table S2). To quantify further the pace of phenotypic change, we estimated evolutionary rates in size at maturation for both periods. The estimated rates in Darwin numerators are positive before (0.28–0.55), but negative after (0.28–0.68) the disease outbreak in both basins and sexes (see electronic supplementary material, table S3).
4. Discussion
We found strong support for our hypothesis that the dramatic change in size-selective mortality in the perch population caused by the pathogen infection (figure 1) led to a rapid response in life-history evolution by reversing the trend in the underlying maturation schedule, from a previously increasing to a decreasing size at maturation (figure 2). In a previous study, Edeline et al. [26] showed that the expansion of the pathogen into the lake was associated with a shift from a competitive to a prey–predator interaction between perch and pike, presumably owing to a reduction in perch body size. Here, we demonstrate that these changes were associated with a shift to earlier maturation, consistent with the hypothesis that the observed ecological changes were driven by an evolutionary response to the pathogen-induced selection. The continuous negative trends over roughly two decades in both basins of the lake further indicate an evolutionary rather than a purely plastic change in the maturation schedule. Moreover, the 1–5 year lag in the response to the pathogen is in agreement with a pathogen-induced evolutionary change in size at maturation as it would be expressed among the maturing offspring of the parents that experienced the selective impact of the pathogen.
The increase in size at maturation prior to the disease is interpreted in two ways: first, as a result of increasing predation pressure from pike that selects against small perch in Windermere [26]; and, second, as a recovery from a commercial fishery on perch that was exceptionally high just before the start of our time series, and finally ceased in 1948 in the north and in 1965 in the south basin [28]. A similar recovery has been reported in Atlantic cod (Gadus morhua) after initiation of a moratorium on the cod fishery [16]. The faster increase in size at maturation in females compared with males during this early period may result from sex-specific selection on body size. Reproductive success generally increases faster with size in females owing to a steeper size–fecundity relationship, whereas, in males, agility and mating behaviour are more important determinants of reproductive success [34]. In contrast, sex-specific responses to a pathogen infection are expected if mortality rates differ between the sexes. Indeed, the decrease in size at maturation after the disease outbreak is more pronounced in males (figure 2), which experience higher pathogen-induced mortality rates than females [35], probably owing to their higher activity and stronger tendency to aggregate on the spawning grounds, and hence a higher risk of infection.
There is growing evidence that evolutionary change in natural populations occurs on time scales similar to those of ecological dynamics [15,36,37]. These observations suggest that it is critical to consider how the temporal dynamics in the ecology of a population are at the same time changing as a result of rapid evolution [38]. Artificial selection from human harvesting has been advocated as a particularly strong agent of rapid phenotypic change [18]. The present study suggests that evolutionary rates caused by natural selection from infectious diseases are comparable to those observed in harvested populations. The estimated rates of 0.28–0.68 (Darwin numerators; see [33]) are slightly higher than a recently suggested average rate of phenotypic change caused by human harvesting, but within the range of reported values [18]. The selective pressure from an infectious disease can be remarkable, as is illustrated by the shift in the population age structure of perch in this study (figure 1). Disease-induced phenotypic change in size at maturation, as reported here (figure 2), may have severe impacts on population abundance, dynamics, recovery and management through altering (i) host–parasite dynamics [39], (ii) the likelihood of population decline and species extinction [2,40], (iii) the population recovery potential [41,42], (iv) the structuring of food webs via interspecific interactions [26,43], and (v) the success of disease and ecosystem management [3].
Our study emphasizes the invaluable scientific benefit of long-term monitoring. Using a probabilistic maturation reaction norm approach, we are able to estimate a temporal trend in size at maturation of perch over a 60-year time period that comprises a severe outbreak of a perch-specific pathogen. We acknowledge that the approach does not necessarily detect purely genetic changes per se, but that other phenotypically plastic responses not related to growth, age or size may contribute to changes in size at maturation [17,44]. However, we statistically control for factors other than growth that are known to influence the life history and show that these variables do not alter the estimated temporal trends in the maturation schedule. Furthermore, by constructing synthetic datasets that simulate the biases in our catch data, we prove that the method estimates the maturation reaction norm correctly and that the estimates are independent of the temporal trend in cohort-specific growth rate.
We conclude that our results strongly suggest an evolutionary response in the maturation schedule of Windermere perch after the disease outbreak in 1976 as a result of the size- and stage-selective mortality on larger, mature fish. The similar temporal trends in the two genetically distinct populations of the two basins further support that these changes reflect adaptive evolutionary changes. The pace at which these evolutionary changes occur indicates that pathogens are highly potent drivers of phenotypic evolution in vertebrates, and should be accounted for in models of infectious diseases and population dynamics. These findings are of particular interest given the current global increase in pathogen incidence.
Acknowledgements
We thank Leif Christian Stige and Geir Storvik for helpful statistical advice. Yannis Michalakis and an anonymous reviewer provided helpful comments on an earlier version of the manuscript. We are also grateful to the Freshwater Biological Association for their joint stewardship of the long-term Windermere data. This work was supported by the Research Council of Norway and the Natural Environment Research Council of the UK.
References
- 1.Harvell C. D., et al. 1999Review: marine ecology—emerging marine diseases—climate links and anthropogenic factors. Science 285, 1505–1510 10.1126/science.285.5433.1505 (doi:10.1126/science.285.5433.1505) [DOI] [PubMed] [Google Scholar]
- 2.Harvell C. D., Mitchell C. E., Ward J. R., Altizer S., Dobson A. P., Ostfeld R. S., Samuel M. D.2002Ecology—climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2162 10.1126/science.1063699 (doi:10.1126/science.1063699) [DOI] [PubMed] [Google Scholar]
- 3.Palumbi S. R.2001Evolution—humans as the world's greatest evolutionary force. Science 293, 1786–1790 10.1126/science.293.5536.1786 (doi:10.1126/science.293.5536.1786) [DOI] [PubMed] [Google Scholar]
- 4.Price P. W.1980Evolutionary biology of parasites. Princeton, NJ: Princeton University Press [Google Scholar]
- 5.Grenfell B. T., Dobson A. P.1995Ecology of infectious diseases in natural populations. Cambridge, UK: Cambridge University Press [Google Scholar]
- 6.Chadwick W., Little T. J.2005A parasite-mediated life-history shift in Daphnia magna. Proc. R. Soc. B 272, 505–509 10.1098/rspb.2004.2959 (doi:10.1098/rspb.2004.2959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan A. B., Little T. J.2007Parasite-driven genetic change in a natural population of Daphnia. Evolution 61, 796–803 10.1111/j.1558-5646.2007.00072.x (doi:10.1111/j.1558-5646.2007.00072.x) [DOI] [PubMed] [Google Scholar]
- 8.Fredensborg B. L., Poulin R.2006Parasitism shaping host life-history evolution: adaptive responses in a marine gastropod to infection by trematodes. J. Anim. Ecol. 75, 44–53 10.1111/j.1365-2656.2005.01021.x (doi:10.1111/j.1365-2656.2005.01021.x) [DOI] [PubMed] [Google Scholar]
- 9.Jones M. E., Cockburn A., Hamede R., Hawkins C., Hesterman H., Lachish S., Mann D., McCallum H., Pemberton D.2008Life-history change in disease-ravaged Tasmanian devil populations. Proc. Natl Acad. Sci. USA 105, 10 023–10 027 10.1073/pnas.0711236105 (doi:10.1073/pnas.0711236105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lafferty K. D.1993The marine snail, Cerithidea californica, matures at smaller sizes where parasitism is high. Oikos 68, 3–11 10.2307/3545303 (doi:10.2307/3545303) [DOI] [Google Scholar]
- 11.Mitchell S. E., Read A. F., Little T. J.2004The effect of a pathogen epidemic on the genetic structure and reproductive strategy of the crustacean Daphnia magna. Ecol. Lett. 7, 848–858 10.1111/j.1461-0248.2004.00639.x (doi:10.1111/j.1461-0248.2004.00639.x) [DOI] [Google Scholar]
- 12.Hochberg M. E., Michalakis Y., de Meeus T.1992Parasitism as a constraint on the rate of life-history evolution. J. Evol. Biol. 5, 491–504 10.1046/j.1420-9101.1992.5030491.x (doi:10.1046/j.1420-9101.1992.5030491.x) [DOI] [Google Scholar]
- 13.Gandon S., Agnew P., Michalakis Y.2002Coevolution between parasite virulence and host life-history traits. Am. Nat. 160, 374–388 10.1086/341525 (doi:10.1086/341525) [DOI] [PubMed] [Google Scholar]
- 14.Reznick D. A., Bryga H., Endler J. A.1990Experimentally induced life-history evolution in a natural population. Nature 346, 357–359 10.1038/346357a0 (doi:10.1038/346357a0) [DOI] [Google Scholar]
- 15.Reznick D. N., Shaw F. H., Rodd F. H., Shaw R. G.1997Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata). Science 275, 1934–1937 10.1126/science.275.5308.1934 (doi:10.1126/science.275.5308.1934) [DOI] [PubMed] [Google Scholar]
- 16.Olsen E. M., Heino M., Lilly G. R., Morgan M. J., Brattey J., Ernande B., Dieckmann U.2004Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature 428, 932–935 10.1038/nature02430 (doi:10.1038/nature02430) [DOI] [PubMed] [Google Scholar]
- 17.Dieckmann U., Heino M.2007Probabilistic maturation reaction norms: their history, strengths, and limitations. Mar. Ecol. Prog. Ser. 335, 253–269 10.3354/meps335253 (doi:10.3354/meps335253) [DOI] [Google Scholar]
- 18.Darimont C. T., Carlson S. M., Kinnison M. T., Paquet P. C., Reimchen T. E., Wilmers C. C.2009Human predators outpace other agents of trait change in the wild. Proc. Natl Acad. Sci. USA 106, 952–954 10.1073/pnas.0809235106 (doi:10.1073/pnas.0809235106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grift R. E., Rijnsdorp A. D., Barot S., Heino M., Dieckmann U.2003Fisheries-induced trends in reaction norms for maturation in North Sea plaice. Mar. Ecol. Prog. Ser. 257, 247–257 10.3354/meps257247 (doi:10.3354/meps257247) [DOI] [Google Scholar]
- 20.Heino M., Dieckmann U., Godø O. R.2002Measuring probabilistic reaction norms for age and size at maturation. Evolution 56, 669–678 [DOI] [PubMed] [Google Scholar]
- 21.Barot S., Heino M., O'Brien L., Dieckmann U.2004Estimating reaction norms for age and size at maturation when age at first reproduction is unknown. Evol. Ecol. Res. 6, 659–678 [Google Scholar]
- 22.Olsen E., Lilly G. R., Heino M., Morgan M. J., Brattey J., Dieckmann U.2005Assessing changes in age and size at maturation in collapsing populations of Atlantic cod (Gadus morhua). Can. J. Fish Aquat. Sci. 62, 811–823 10.1139/f05-065 (doi:10.1139/f05-065) [DOI] [Google Scholar]
- 23.Kipling C., Le Cren E. D.1984Mark-recapture experiments on fish in Windermere, 1943–1982. J. Fish Biol. 24, 395–414 10.1111/j.1095-8649.1984.tb04811.x (doi:10.1111/j.1095-8649.1984.tb04811.x) [DOI] [Google Scholar]
- 24.Bodaly R. A., Ward R. D., Mills C. A.1989A genetic stock study of perch, Perca fluviatilis L., in Windermere. J. Fish Biol. 34, 965–967 10.1111/j.1095-8649.1989.tb03380.x (doi:10.1111/j.1095-8649.1989.tb03380.x) [DOI] [Google Scholar]
- 25.Bucke D., Cawley G. D., Craig J. F., Pickering A. D., Willoughby L. G.1979Further studies of an epizootic of perch, Perca fluviatilis L., of uncertain aetiology. J. Fish Dis. 2, 297–311 10.1111/j.1365-2761.1979.tb00172.x (doi:10.1111/j.1365-2761.1979.tb00172.x) [DOI] [Google Scholar]
- 26.Edeline E., Ben Ari T., Vøllestad L. A., Winfield I. J., Fletcher J. M., Ben James J., Stenseth N. C.2008Antagonistic selection from predators and pathogens alters food-web structure. Proc. Natl Acad. Sci. USA 105, 19 792–19 796 10.1073/pnas.0808011105 (doi:10.1073/pnas.0808011105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pickering A. D., Willoughby L. G.1977Epidermal lesions and fungal infection on the perch, Perca fluviatilis L., in Windermere. J. Fish Biol. 11, 349–354 10.1111/j.1095-8649.1977.tb04128.x (doi:10.1111/j.1095-8649.1977.tb04128.x) [DOI] [Google Scholar]
- 28.Le Cren E. D., Kipling C., McCormack J. C.1977Study of numbers, biomass and year-class strengths of perch (Perca fluviatilis L.) in Windermere from 1941 to 1966. J. Anim. Ecol. 46, 281–307 [Google Scholar]
- 29.Le Cren E. D.1951The length–weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 20, 201–219 [Google Scholar]
- 30.Manly B. F. J.2007Randomization, bootstrap and Monte Carlo methods in biology. Boca Raton, FL: Chapman & Hall [Google Scholar]
- 31.R Development Core Team 2007R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 32.Muggeo V. M. R.2008Segmented: an R package to fit regression models with broken-line relationships. R News 8, 20–25 [Google Scholar]
- 33.Hendry A. P., Kinnison M. T.1999The pace of modern life: measuring rates of contemporary microevolution. Evolution 53, 1637–1653 10.2307/2640428 (doi:10.2307/2640428) [DOI] [PubMed] [Google Scholar]
- 34.Fairbairn D. J., Blanckenhorn W. U., Szekeley T.2007Sex, size and gender roles: evolutionary studies of sexual size dimorphism. Oxford, UK: Oxford University Press [Google Scholar]
- 35.Paxton C. G. M., Fletcher J. M., Hewitt D. P., Winfield I. J.1999Sex ratio changes in the long-term Windermere pike and perch sampling program. Ecol. Freshw. Fish 8, 78–84 10.1111/j.1600-0633.1999.tb00057.x (doi:10.1111/j.1600-0633.1999.tb00057.x) [DOI] [Google Scholar]
- 36.Grant P. R., Grant B. R.2002Unpredictable evolution in a 30-year study of Darwin's finches. Science 296, 707–711 10.1126/science.1070315 (doi:10.1126/science.1070315) [DOI] [PubMed] [Google Scholar]
- 37.Yoshida T., Jones L. E., Ellner S. P., Fussmann G. F., Hairston N. G.2003Rapid evolution drives ecological dynamics in a predator–prey system. Nature 424, 303–306 10.1038/nature01767 (doi:10.1038/nature01767) [DOI] [PubMed] [Google Scholar]
- 38.Hairston N. G., Ellner S. P., Geber M. A., Yoshida T., Fox J. A.2005Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett 8, 1114–1127 10.1111/j.1461-0248.2005.00812.x (doi:10.1111/j.1461-0248.2005.00812.x) [DOI] [Google Scholar]
- 39.Duffy M. A., Sivars-Becker L.2007Rapid evolution and ecological host–parasite dynamics. Ecol. Lett. 10, 44–53 10.1111/j.1461-0248.2006.00995.x (doi:10.1111/j.1461-0248.2006.00995.x) [DOI] [PubMed] [Google Scholar]
- 40.Hutchings J. A., Myers R. A.1993Effect of age on the seasonality of maturation and spawning of Atlantic cod, Gadus morhua, in the Northwest Atlantic. Can. J. Fish Aquat. Sci. 50, 2468–2474 10.1139/f93-271 (doi:10.1139/f93-271) [DOI] [Google Scholar]
- 41.Walsh M. R., Munch S. B., Chiba S., Conover D. O.2006Maladaptive changes in multiple traits caused by fishing: impediments to population recovery. Ecol. Lett. 9, 142–148 10.1111/j.1461-0248.2005.00858.x (doi:10.1111/j.1461-0248.2005.00858.x) [DOI] [PubMed] [Google Scholar]
- 42.Swain D. P., Sinclair A. F., Hanson J. M.2007Evolutionary response to size-selective mortality in an exploited fish population. Proc. R. Soc. B 274, 1015–1022 10.1098/rspb.2006.0275 (doi:10.1098/rspb.2006.0275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood C. L., Byers J. E., Cottingham K. L., Altman I., Donahue M. J., Blakeslee A. M. H.2007Parasites alter community structure. Proc. Natl Acad. Sci. USA 104, 9335–9339 10.1073/pnas.0700062104 (doi:10.1073/pnas.0700062104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kraak S. B. M.2007Does the probabilistic maturation reaction norm approach disentangle phenotypic plasticity from genetic change? Mar. Ecol. Prog. Ser. 335, 295–300 10.3354/meps335295 (doi:10.3354/meps335295) [DOI] [Google Scholar]