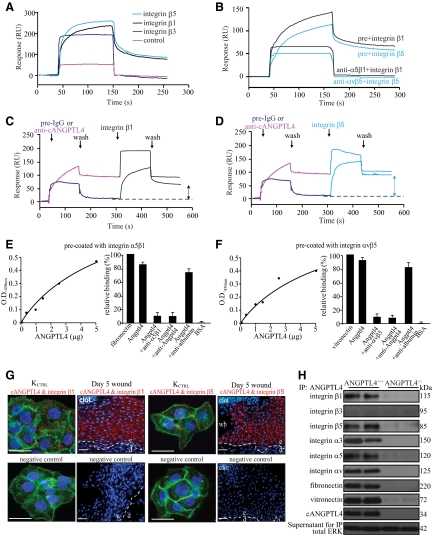
Figure 5.
ANGPTL4 interacts with integrins β1 and β5. Representative sensorgrams showing binding profiles between immobilized cANGPTL4 and S2-membrane extracts containing either control, integrin β1, β3, or β5 (A), or integrin β1 or β5 preincubated with either preimmune IgG (pre) or cognate anti-integrin antibody (B). Integrin β1 (C) or integrin β5 (D) after preblocked with either preimmune IgG or anti-cANGPTL4 antibody. Each sensorgram was corrected by subtracting a sensorgram obtained from a reference flow cell with no immobilized protein. Anti-cANGPTL4 antibodies against the immobilized cANGPTL4 determined the Rmax value to be 138.2 resonance units (RU). Five independent experiments were performed. Dose-dependent ANGPTL4 binding to immobilized (E) integrin α5β1 or (F) integrin αvβ5, which was specifically blocked by anti-cANGPTL4, as determined by ELISA. Detection of (G) the ANGPTL4-integrin β1 (left panel) and ANGPTL4-integrin β5 (right panel) complexes in KCTRL and in day-5 ANGPTL4+/+ wound biopsies using DUOlink PLA. PLA signals (red) and Hoechst dye for nuclei (blue). For KCTRL, the cells were counterstained with Alexa 488-phallodin for actin stress fibers. The nuclear image was acquired in one z-plane using a LSM510 META confocal laser-scanning microscope (Carl Zeiss). Dotted white line represents epidermal-dermal junction. Negative control was performed without primary antibodies. Representative pictures from wound sections with epidermis (e), dermis (d), wound bed (wb), and KCTRL from six independent experiments or sections from three mice are shown. Scale bar = 40 μm. H: Immunodetection of indicated proteins from anti-cANGPTL4 immunoprecipitates of ANGPTL4+/+ and ANGPTL4−/− wound biopsy homogenates. Total ERK from supernatant were used to verify equal loading.