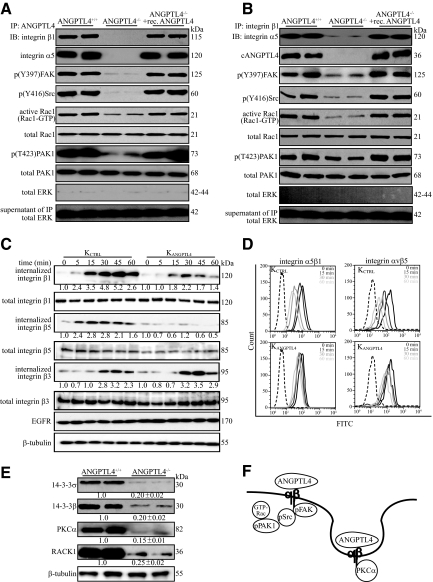
Figure 6.
ANGPTL4 modulates integrin-mediated signaling and internalization. Immunodetection of indicated proteins in anti-cANGPTL4 (A) or anti-integrin β1 immunoprecipitates (B) (top panel); of Rac1-GTP and phosphorylated PAK1 (p(T423)PAK1) (lower panels) from membrane extract of indicated wound biopsies. Total PAK from total cell lysate were used to verify equal loading. Specificity of immunoprecipitation was verified by immunodetection of ERK in the immunoprecipitates and its supernatant. C: Kinetics of integrin internalization. Internalized biotinylated-integrins were detected using corresponding antibodies after immunoprecipitation with NeutrAvidin agarose resins. The level of total integrins β1, β3, and β5 were determined using total cell lysate before immunoprecipitation. EGFR and β-tubulin from total cell lysate were used to verify equal loading. Values denote mean fold change of three independent experiments compared to KCTRL at time 0. D: Cell-surface expression of the integrins α5β1 and αvβ5 in KCTRL and KANGPTL4 at the indicated time was determined by FACS. The negative control (only secondary antibody) is indicated by the dotted graph. E: Immunoblot analysis of membrane extracts from day-5 ANGPTL4+/+ and ANGPTL4−/− wound biopsies for indicated proteins. Values below the band represent the mean fold differences in protein expression levels relative to ANGPTL4+/+ from eight wound biopsies for each genotype. β-tubulin was used as loading and transfer control. F: Schematic illustration showing ANGPTL4 interacting with integrin, activating FAK-Src-PAK1 signaling and facilitating integrin internalization, which involves PKCα and 14-3-3σ/β, to aid cell migration.