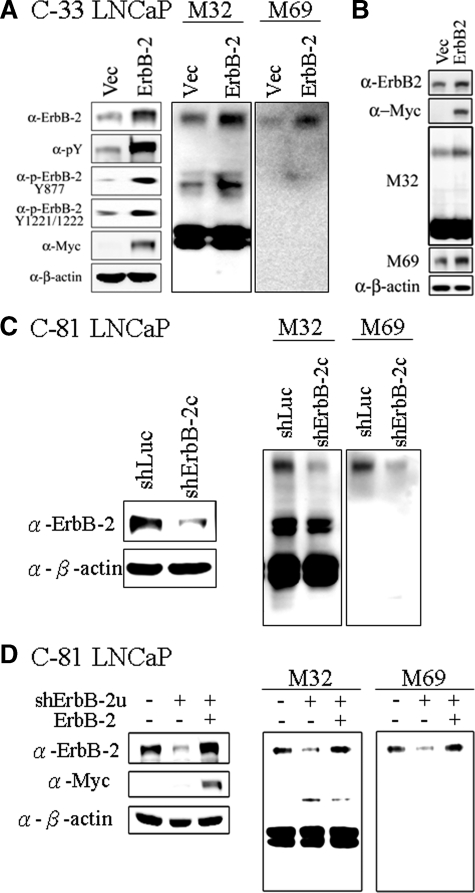
Figure 3.
Effects of ErbB-2 level and activity on matriptase zymogen activation in PCa cells. A: Effects of ErbB-2 overexpression on matriptase activation in C-33 LNCaP cells. LNCaP C-33 cells were transiently transfected with ErbB-2 cDNA by Lipofectamine 2000 reagents, and control cells were transiently transfected with vector alone. Two days after transfection, cells were harvested for Western blot analysis. The separated proteins were transferred to a NC membrane and detected by immunoblotting with anti-pTyr (PY100), anti-ErbB-2 (C18), anti-c-Myc, and anti-β-actin (AC-15) Abs. For analyses of matriptase and HAI-1, nonreduced and nonboiled cell lysates were collected with Triton 1% X-100 in PBS, and immunoblotted with anti-total matriptase (M32) and anti-activated matriptase (M69) mAbs. B: Effects of ErbB-2 overexpression on matriptase activation in DU 145 cells. DU 145 cells were transiently transfected with ErbB-2 cDNA, and the immunoblots were performed as described in A. C: Effects of ErbB-2 knockdown on activation of matriptase in C-81 LNCaP cells. Cells were seeded at a density of 1.2 × 106 per well in 6-cm dishes. One day after plating, cells were infected with lentiviral particles containing ErbB-2 shRNA for 24 hours. Control cells were infected with lentiviral particles containing luciferase shRNA. Three days after selection with 1 μg/ml puromycin, cells were harvested for Western blot analysis. For analyses of the p-Tyr and protein levels of ErbB-2, cell lysates were collected and detected by anti-pTyr (PY100) and anti-ErbB-2 (C18) Abs. For analyses of matriptase and HAI-1, nonreduced and nonboiled cell lysates were collected with Triton 1% X-100 in PBS. Matriptase and HAI-1 were detected by immunoblotting with anti-total matriptase (M32) and anti-activated matriptase (M69) mAbs. Equal protein loading was verified by blotting the membranes with an anti-β-actin Ab (AC-15). D: Effects of ErbB-2 re-expression on matriptase activation in ErbB-2-knockdown cells. C-81 LNCaP cells were infected with viral particles with control luciferase shRNA or ErbB-2u shRNA for 3 days. The ErbB-2u shRNA was designed to target a specific sequence located in the 3′ UTR of ErbB-2. The ErbB-2- knockdown cells were transfected with control vector or ErbB-2 cDNA and cultured for 3 days. Cell lysates were collected and assayed by using an anti-ErbB-2 Ab (C18). For analyses of matriptase, cell lysates were collected as described in Figure 1D to detect the levels of total and activated matriptase by immunoblotting with anti-total matriptase (M32) and anti-activated matriptase (M69) mAbs. Equal protein loading was verified by blotting the membranes with an anti-β-actin Ab (AC-15).