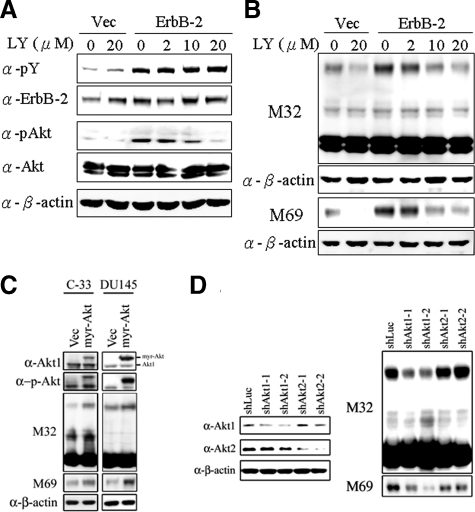
Figure 5.
Role of PI 3 kinases/Akt in ErbB-2-induced matriptase activation in C-33 LNCaP cells. Stable ErbB-2-overexpressing C-33 LNCaP cells were seeded at a density of 6 × 105 per well in a 6-well plate. Two days after plating, cells were treated with LY294002 at concentrations of 0, 2, 10, and 20 μmol/L; control cells were treated with concentrations of 0 and 20 μmol/L PD98059. Treatment was carried out for 24 hours, and then cells were harvested for Western blot analysis. A: Cell lysates were collected with 0.5% NP-40 in HEPES buffer and evaluated by immunoblots for p-Tyr and protein levels of ErbB-2, performed as described previously. The phosphorylation and protein levels of Akt were detected by anti-phosphoAkt (Ser473) and anti-Akt1/2 Abs. B: Nonreduced and nonboiled cell lysates were used for immunoblotting to detect the activation status and total matriptase with anti-total matriptase (M32) and anti-activated matriptase (M69) mAbs. An anti-β-actin (AC-15) Ab was used to evaluate protein loading. C: Role of a constitutively activated Akt (Myr-Akt) in matriptase zymogen activation in C-33 LNCaP cells and DU 145 cells. LNCaP C-33 cells and DU 145 cells were transiently transfected with Myr-Akt and control plasmids by using Lipofectamine 2000. Two days after transfection, cell lysates were collected and analyzed by immunoblotting with anti-phosphoAkt (Ser473), anti-Akt1/2, anti-total matriptase (M32), and anti-activated matriptase (M69) Abs, respectively. D: Effect of Akt1 or Akt2 knockdown on activation of matriptase in LNCaP C-81 cells. Cells were seeded at a density of 1.2 × 106 per well in 6-cm dishes. One day after plating, cells were transfect with Akt1 shRNAs (shAkt1-1 and shAkt1-2), Akt2 shRNAs (shAkt2-1 and shAkt2-2), and control luciferase shRNA with Lipofectamine 2000. Cell lysates were collected for analysis of the levels of Akt1 and Akt2 protein by immunoblot with anti-Akt1 and anti-Akt2 Abs. For analyses of matriptase, cell lysates were collected and immunoblotted with anti-total matriptase (M32) and anti-activated matriptase (M69) mAbs. Equal protein loading was assessed with an anti-β-actin Ab (AC-15).