Abstract
Although an immunoregulatory role of aryl hydrocarbon receptor (Ahr) has been demonstrated in T cells and macrophages, little is known about its function in dendritic cells (DC). Here, we show that lipopolysaccharide (LPS) and CpG stimulate Ahr expression in bone marrow-derived dendritic cells (BMDC). Furthermore, we found that Ahr is required to induce indoleamine 2,3-dioxygenase (IDO) expression, an immunosuppressive enzyme that catabolizes tryptophan into kynurenine (Kyn) and other metabolites in DC. In the presence of LPS or CpG, Ahr-deficient (Ahr−/−) mature BMDC induced immune responses characterized by reduced Kyn and IL-10 production compared with results observed with tolerogenic mature WT BMDC. In a coculture system with LPS- or CpG-stimulated BMDC and naive T cells, Ahr−/− BMDC inhibited naive T-cell differentiation into regulatory T (Treg) cells, which likely facilitated Th17 cell development and promoted naive T-cell proliferation. Addition of synthetic L-Kyn to the coculture system skewed the differentiation of naive T cells to Treg cells rather than Th17 cells. Taken together, our results demonstrate a previously unknown negatively regulatory role for Ahr in DC-mediated immunogenesis in the presence of LPS or CpG, which, in turn, alters the Kyn-dependent generation of Treg cells and Th17 cells from naive T cells.
Keywords: dioxin receptor, tryptophan catabolism, immune regulation
The precise regulatory mechanisms governing the generation of regulatory T (Treg) cells and IL-17–producing helper T (Th17) cells in response to Toll-like receptor (TLR) activation are not fully understood. Differentiation of naive T cells into Treg and Th17 cells is regulated by a combination of extracellular cytokines and intracellular transcription factors. Aryl hydrocarbon receptor (Ahr), a ligand-activated transcription factor that mediates dioxin toxicity, has recently emerged as an important factor in the regulation of immune responses (1). Ahr belongs to the PER-ARNT-SIM superfamily of proteins (2, 3). Cytoplasmic inactive Ahr forms a complex with the chaperone Hsp90 and the cochaperones Ahr-interacting protein and phosphoprotein p23 (4–6). Ahr ligands, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), diffuse into the cell and bind to the cytosolic Ahr complex, leading to translocation of the ligand−Ahr complex into the nucleus. In the nucleus, ligand−Ahr complexes dimerize with Ahr nuclear translocator and bind to dioxin response elements in the promoters of target genes, including those that encode cytochrome family proteins and Ahr repressor (7, 8). Ahr activation by TCDD causes various toxic responses, including cellular damage and carcinogenesis (9, 10). It was previously reported that Ahr activation by TCDD or the UV photoproduct of tryptophan 6-formylindolo[3,2-b]carbazole induces the development of Foxp3+ Treg or Th17 cells, respectively (11–13). In line with these results, our group demonstrated that Ahr activation in T cells by IL-6 plus TGF-β and in macrophages by such TLR ligands as lipopolysaccharide (LPS) promotes Th17 cell differentiation by regulating the activation of signal transducer and activator of transcription 1 (Stat1) and the expression of proinflammatory cytokines, including IL-6, TNF-α, and IL-12 (14, 15).
In addition to macrophages, dendritic cells (DC) serve as professional antigen-presenting cells (APC) that induce the differentiation of naive T cells into effector T cells (16). The immunoregulatory roles of Ahr in DC, however, are not fully understood. Among the specialized subsets of DC, regulatory DC (DCreg) play a pivotal role in immune responses by regulating a complex cytokine network. DCreg can be divided into immunogenic and tolerogenic DC, although no distinguishing marker is currently available (17). The functions of DCreg in T-cell activation are mainly mediated by regulatory factors, such as IL-10 and indoleamine 2,3-dioxygenase (IDO) (18). IDO is an immunosuppressive enzyme that consumes oxygen and catalyzes the essential amino acid tryptophan (Trp) into kynurenine (Kyn) (Fig. S1). These subsequent metabolites along this metabolic pathway—collectively referred to as kynurenines—are created by downstream enzymes that are specifically expressed in different cell types (19). IDO activity is induced in APC by IFN-γ, LPS, and CpG (20–25). The resulting reduction in Trp levels limits the growth of microorganisms and inhibits T-cell proliferation. In addition, Kyn causes apoptosis of effector T cells, most notably Th1 cells (26–28), whereas the combined effect of Trp starvation and Kyn production induces Treg cell development (29).
Previous reports showed that Ahr signaling is required for the expression of IDO in DC (30, 31). These studies, however, did not examine how Ahr in DC regulates the differentiation of Th cell lineages, such as Treg and Th17 cells. In addition, aromatic amino acids (e.g., Trp) can serve as Ahr agonists to promote Th17 differentiation (32). These studies suggest that interactions between Ahr and IDO are crucial for modulating immune responses. Interestingly, kynurenines have been demonstrated to be effective in the treatment of several Treg cell-mediated autoimmune conditions, such as experimental murine autoimmune encephalomyelitis and collagen-induced arthritis (33, 34). The roles of Ahr-induced signaling in these murine disease models are, however, unknown.
In the current study, we test the hypothesis that LPS and CpG activate Ahr in bone marrow-derived dendritic cells (BMDC), and thereby regulate IDO expression and DC function to drive the differentiation of naive T cell into Treg and Th17 cells.
Results
Ahr Is Expressed in Response to LPS or CpG Stimulation in BMDC.
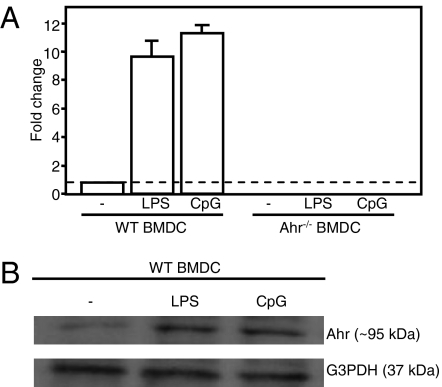
We previously reported that Ahr contributes to Th17 cell generation from naive T cells that have been stimulated with IL-6 and TGF-β (14). In macrophages, Ahr expression is induced by LPS stimulation, and an absence of the protein in Ahr−/− mice augments IL-6 levels, leading to increased LPS sensitivity (15). The roles of Ahr in DC, however, are not fully understood. Therefore, we investigated Ahr expression in DC in the presence of TLR4-LPS or TLR9-CpG signaling. BMDC from WT mice were stimulated with LPS or CpG for 24 h. Quantitative real-time PCR (qPCR) and Western blot analysis revealed that Ahr was not constitutively expressed in BMDC, whereas both LPS and CpG stimulated Ahr expression in these cells (Fig. 1 A and B). The Ahr was also expressed in splenic DC stimulated with LPS or CpG for 24 h (Fig. S2B).
Fig. 1.
LPS and CpG stimulated Ahr expression in BMDC. Bone marrow cells were cultured in the presence of GM-CSF for 9 d. MACS-sorted CD11c+ BMDC were then harvested and stimulated with LPS or CpG for 24 h. (A) Ahr mRNA expression was examined using qPCRs. (B) Cells were lysed and analyzed by Western blotting for Ahr and G3PDH. Representative data were obtained from one of two experiments.
IL-10 Production Was Reduced in Ahr−/− BMDC Stimulated with LPS or CpG.
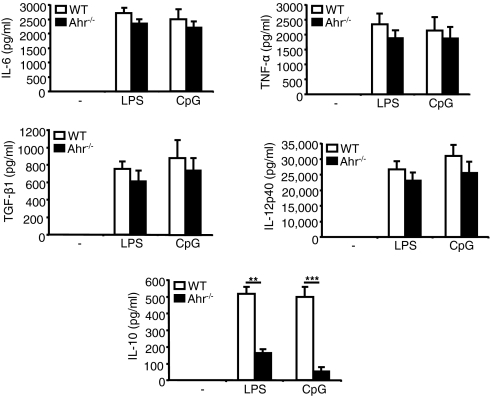
Next, we used ELISAs to examine the profile of cytokine production in WT and Ahr−/− BMDC stimulated with LPS or CpG for 24 h. Fig. 2 shows that the levels of such inflammatory cytokines as IL-6, TNF-α, IL-12p40, and TGF-β did not differ between the WT and Ahr−/− samples. Interestingly, the level of IL-10, an anti-inflammatory cytokine, was significantly reduced in Ahr−/− BMDC treated with LPS or CpG, compared with results observed in WT BMDC. The level of IL-10 was dramatically declined in Ahr−/− splenic DC treated with LPS or CpG, compared with results observed in WT splenic DC (Fig. S2C). It is likely that the absence of Ahr suppressed a regulatory function of DCreg by inhibiting IL-10 production in response to LPS or CpG stimulation.
Fig. 2.
IL-10 production was inhibited in Ahr−/− BMDC stimulated with LPS or CpG. WT and Ahr−/− BMDC were stimulated with LPS or CpG and culture supernatants were harvested after 24 h. Levels of IL-6, TNF-α, IL-12p40, TGF-β1, and IL-10 were measured using ELISA. Data show mean ± SD from three independent experiments. **P < 0.01; ***P < 0.005.
IDO Was Not Expressed in Ahr−/− BMDC in Response to LPS or CpG Stimulation.
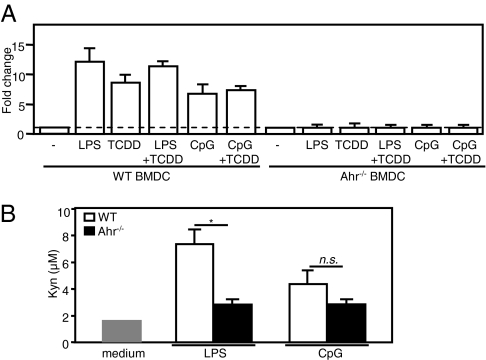
Expression of functional IDO is induced in response to IFN-γ, LPS, and CpG in macrophages and several subsets of DC, including myeloid BMDC (23, 35–37). TCDD-induced Ahr expression was recently reported to mediate IDO expression in DC (30). Therefore, we asked whether IDO expression in BMDC in response to LPS or CpG stimulation depended on Ahr expression. To answer this question, we used qPCRs to assay IDO mRNA expression in WT and Ahr−/− BMDC stimulated with either LPS or CpG alone or in combination with TCDD for 24 h. As shown in Fig. 3A, TCDD, LPS, or CpG stimulated IDO mRNA expression in WT BMDC. The increased IDO expression observed in WT BMDC was completely suppressed in Ahr−/− BMDC stimulated with LPS or CpG. LPS or CpG was able to stimulate IDO mRNA expression in WT splenic DC but not in Ahr−/− splenic DC (Fig. S2D).
Fig. 3.
IDO was not expressed in Ahr−/− BMDC stimulated with LPS or CpG. (A) WT and Ahr−/− BMDC were stimulated with LPS or CpG either alone or in combination with TCDD for 24 h. IDO mRNA expression was examined using qPCR. (B) To measure Kyn levels, WT and Ahr−/− BMDC were cultured in complete RPMI medium 1640 plus L-Trp (100 μM). Cells were then stimulated with LPS or CpG, and culture supernatants were harvested after 18 h. Kyn levels were measured in culture supernatants using a colorimetric assay. Data show mean ± SD from three independent experiments. *P < 0.05; n.s., not significant.
IFN-γ and TLR-4 ligands, such as LPS, induce functional IDO expression in BMDC (17). We verified the activity of IDO by measuring Kyn levels in cell culture supernatant using a colorimetric method. WT and Ahr−/− BMDC were stimulated with LPS or CpG, and culture supernatants were harvested after 18 h. As shown in Fig. 3B, the levels of Kyn in culture supernatant of WT BMDC stimulated with LPS were higher than that in culture supernatant of Ahr−/− BMDC. In a previous study, Kyn was not detected in culture supernatant of WT BMDC stimulated with CpG due to high-affinity uptake of BMDC to Kyn (38). We detected a low level of Kyn in culture supernatant of CpG-stimulated WT BMDC, however.
Ahr in DC Regulates the Generation of Treg and Th17 Cells from Naive T Cells.
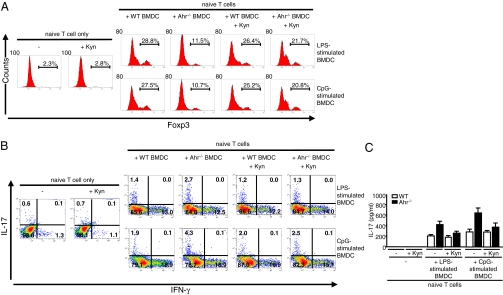
IDO+ DC play an important role in immune tolerance, a process that involves Treg cell development (20, 21). We previously reported that an absence of Ahr in T cells significantly impaired Treg cell development (14). Here, we determined whether Ahr activation in DC participates in the differentiation of naive T cells into various Th cell lineages. To accomplish this goal, we used an in vitro BMDC–T cell-coculture system. WT and Ahr−/− BMDC were stimulated with LPS or CpG for 24 h. Naive T cells were isolated from WT mice and cocultured with the stimulated WT and Ahr−/− BMDC. After 4 d, Foxp3 expression in T cells was analyzed by FACS. Foxp3 expression was significantly suppressed in T cells that were activated by Ahr−/− BMDC compared with results observed in T cells activated by WT BMDC (Fig. 4A).
Fig. 4.
Absence of Ahr in BMDC inhibited Treg development and facilitated Th17 cell generation from naive T cells. WT and Ahr−/− BMDC were stimulated with LPS or CpG for 24 h. MACS-sorted naive T cells from WT mice were then cocultured with or without stimulated BMDC for 4 d. Synthetic L-Kyn (50 μM) was added to some samples as indicated. (A) After 4 d coculture, Foxp3 expression in T cells was examined using FITC-conjugated anti-mouse Foxp3 antibodies and stained cells were analyzed using FACS. (B) After 4 d coculture, T cells were stimulated with PMA/ionomycin for 5 h; GolgiStop was added for the final 2 h. T cells were then intracellularly stained for IL-17 and IFN-γ and analyzed using FACS. Data are shown as dot blots. (C) Levels of IL-17 in coculture supernatants of LPS- or CpG-stimulated WT and Ahr−/− BMDC with naive T cells after 4 d. Supernatants were harvested from BMDC–T-cell coculture after 4 d. IL-17 was measured in coculture supernatants using ELISA. These results are representative of three independent experiments.
The altered development of Treg cells may have affected Th17 and Th1 cell generation from naive T cells. Therefore, we examined the generation of Th17 and Th1 cells by assessing intracellular expression of IL-17 and IFN-γ in T cells. After coculture with BMDC for 4 d, T cells were treated with phorbol 12-myristate 13-acetate (PMA)/ionomycin, and analyzed for intracellular IL-17 and IFN-γ using FACS. More IL-17–producing T cells were observed in cocultures containing Ahr−/− BMDC than in those containing WT BMDC (Fig. 4B).
As shown in Fig. 3B, Kyn levels were reduced in Ahr−/− BMDC. Compared with CpG, LPS stimulated WT BMDC to produce more Kyn. Nevertheless, even the low levels of Kyn produced by CpG-stimulated WT BMDC may regulate DC function. Moreover, Kyn has been shown to restore the regulatory functions of DC lacking functional IDO (39). Thus, we hypothesized that reduced Kyn levels caused by an absence of IDO in LPS- or CpG-stimulated Ahr−/− BMDC inhibit Treg cell generation from naive T cells. To test this hypothesis, synthetic L-Kyn was added to the BMDC–T cell-coculture system. At a concentration of 50 μM, L-Kyn did not affect T-cell viability (Fig. S3). Interestingly, exogenous Kyn reversed the generation of Treg cells from naive T cells. As shown in Fig. 4A, the percentage of Foxp3+ Treg cells increased in cocultures containing Kyn-treated Ahr−/− BMDC rather than untreated Ahr−/− BMDC. The increased number of Treg cells probably caused a reduction in the number of Th17 cells in the Kyn-treated cocultures (Fig. 4B).
We quantified IL-17 levels in coculture supernatant using ELISAs. IL-17 levels were higher in Ahr−/− BMDC–T cell-cocultures compared with WT BMDC–T cell-cocultures (Fig. 4C). Exogenous L-Kyn suppressed the level of IL-17 in Ahr−/− BMDC–T cell-cocultures. Collectively, our results suggest that an absence of Ahr is likely to impair the regulatory activities of DC and consequently to skew naive T-cell differentiation toward Th17 cells and away from Treg cells, possibly via an IDO-related mechanism.
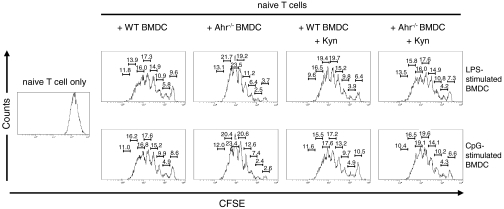
Absence of Ahr in DC Promotes Naive T-Cell Proliferation.
We found that Ahr was involved in regulation of the differentiation of naive T cells into Treg and Th17 cells (Fig. 4). A previous report showed that IDO-induced Trp metabolites in macrophages affect T-cell proliferation (40). Because Ahr is required for IDO expression, we asked whether the proliferation of naive T cells was affected by an absence of Ahr in BMDC stimulated with LPS or CpG. We assessed T-cell proliferation using a carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution assay. Naive T cells were labeled with CFSE and cocultured with LPS- or CpG-stimulated WT or Ahr−/− BMDC. After 4 d coculture, the dilution of CFSE in labeled T cells was determined using FACS analysis. The proliferation of naive T cells was promoted in Ahr−/− BMDC–T cell-cocultures compared with WT BMDC–T cell-cocultures (Fig. 5). Addition of L-Kyn to the Ahr−/− BMDC–T cell-cocultures inhibited naive T-cell proliferation. Thus, our data suggest that an absence of Ahr in DC promotes the proliferation of naive T cells, a process that likely reflects inhibition of Treg cell development and a lack of Kyn production.
Fig. 5.
Absence of Ahr in BMDC promoted naive T-cell proliferation. MACS-sorted naive T cells from WT mice were stained with 3 μM CFSE at 37 °C for 10 min. WT and Ahr−/− BMDC were stimulated with LPS or CpG for 24 h. CFSE-labeled naive T cells were cocultured with or without stimulated BMDC. L-Kyn (50 μM) was added to some samples as indicated. After 4 d, cell proliferation was examined based on CFSE dilution using FACS. Equivalent percentage of proliferation in each generation of cells is shown. These results are representative of three independent experiments.
Discussion
Although Ahr has been studied for many years as a receptor for environmental contaminants, this transcription factor has recently become an attractive research topic for immunologists investigating its roles in the immune system (41). Indeed, several groups have recently revealed significant evidence of a role for Ahr in immune response regulation via dioxin receptors (12–15). Our data here demonstrate a previously unknown role for Ahr in modulating the function of DCreg by inducing naive T-cell differentiation into Treg and Th17 cells in the presence of such pathogenic stimuli as LPS and CpG.
Many endogenous and exogenous ligands activate Ahr, including UV photoproducts of Trp and halogenated dioxin (41). Furthermore, TLR ligands and cytokines are potent Ahr agonists in various cell types. Ahr activated in T cells in response to IL-6 plus TGF-β, and in macrophages in response to LPS stimulation was shown to participate in Th17 cell development (14, 15). Here, we have shown that LPS and CpG stimulate Ahr expression in BMDC (Fig. 1). In mice, immature DC are activated via the TLR-4 ligand LPS or the TLR-9 ligand CpG to become mature DC (42). Mature DC produce cytokines that regulate the differentiation of naive T cells into various Th cell subsets. CD4+ Th cell subsets, such as Treg and Th17 cells, contribute to both tolerance and autoimmunity. Mature BMDC produce such pleiotropic cytokines as IL-6, TNF-α, IL-12, and IL-10 to activate naive T cells. We showed that an absence of Ahr in BMDC or splenic DC impairs the secretion of IL-10 (Fig. 2 and Fig. S2C). We also found that IL-10 production was inhibited in WT BMDC stimulated with LPS or CpG in the presence of such Ahr antagonists as CH-223191 or resveratrol (Fig. S4). We speculated that reduced IL-10 production by Ahr−/− BMDC or splenic DC negatively affects tolerance, shifting conditions to a proinflammatory state. Taken together, the results show that Ahr plays an anti-inflammatory role in BMDC and splenic DC.
The function of Ahr in BMDC and in peritoneal macrophages is different (15). The induction of Ahr expression in BMDC by either LPS or CpG stimulation was similar to results observed in macrophages. However, Ahr deficiency did augment LPS-induced proinflammatory responses in macrophage but not in BMDC (15). The inhibition of IL-10 production was observed in LPS-stimulated Ahr−/− macrophages, BMDC, and splenic DC (15). Interestingly, however, the inhibition of IL-10 production was observed in CpG-stimulated Ahr−/− BMDC and splenic DC but not in CpG-stimulated Ahr−/− peritoneal macrophages.
IDO is expressed in monocytes/macrophages or DC in response to LPS or CpG stimulation to modulate inflammatory responses (37, 43). IDO expression is induced in the presence of TLR ligands or inflammatory cytokines through either an IFN-γ−dependent pathway via STAT1α and IRF-1 or an IFN-γ−independent pathway via NF-κB and p38 MAPK (23). Vogel et al. showed that Ahr expression in response to TCDD mediates IDO expression in DC in vitro or in the spleens and lungs of mice in vivo (30). We have demonstrated that, in addition to LPS and TCDD, CpG also induces IDO expression in WT BMDC but not in Ahr−/− BMDC (Fig. 3A). We further investigated the roles of WT and Ahr−/− BMDC in naive T-cell activation. Trp starvation and kynurenines were previously shown to convert naive T cells into Treg cells, which help to prevent autoimmune disease (29, 44). In this study, we demonstrated that an absence of Ahr in BMDC skews naive T-cell differentiation away from a Treg cell fate (Fig. 4A). Previous reports of the regulatory roles of Ahr in the development of Foxp3+ Treg cells have described inconsistent results. Activation of Ahr concomitant with an increase in the Treg cell population was demonstrated by our group and Quintana et al. (12) and Kimura et al. (14). In a graft-versus-host disease model, however, Marshall et al. showed a decrease in Foxp3+ T-cell numbers during Ahr activation (45). Recently, Mezrich et al. demonstrated that Kyn induced the generation of Treg from naive T cells in an Ahr-dependent manner (46). In our results, however, we found that Ahr−/− BMDC skewed the differentiation of naive T cells toward a Th17 cell fate, possibly due to an inhibition of Treg cell development (Fig. 4B). We hypothesized that inhibition of Treg cell development in the absence of Ahr in DC disrupts Th17 cell generation from naive T cells. Therefore, the Ahr-dependent mechanism that triggers the generation of Treg and Th17 cells requires further study. The role of Ahr in regulating Th17 cell development has been previously reported (13, 14). Th17 cells respond to extracellular bacteria, mediate inflammation, and cause pathologic effects in certain autoimmune diseases. Imbalance in the ratio of Treg cells to Th17 cells dysregulates lymphocyte activation and immune responses, which may contribute to various autoimmune disorders. Moreover, inhibition of Treg cell development due to an absence of Ahr in DC seems to promote naive T-cell proliferation (Fig. 5).
Deficiencies in IDO activity impair Trp degradation, and consequently disrupt immune tolerance (43). IDO activates Treg cells and inhibits the conversion of Treg cells into Th17-like cells (36). Romani et al. suggested that kynurenines produced via IDO negatively regulate IL-17 and IL-23 levels and inhibit autoimmunity via IL-17 signaling (47). On the other hand, IDO has been shown to drive autoimmune responses via B cells (48). Thus, IDO may contribute to both tolerance and autoimmunity depending on the type of immune cells. Published evidence suggests that IDO-induced Trp metabolites are Ahr ligands that mediate the development of Treg cells (29, 49). In our BMDC–T-cell coculture system, synthetic L-Kyn inhibited naive T-cell differentiation into Treg cells.
In summary, we have identified a previously uncharacterized role for Ahr in regulating tolerogenic or immunogenic activities of DC in the presence of LPS or CpG. We have also demonstrated that an absence of Ahr in stimulated BMDC skews naive T-cell differentiation toward Th17 cells and away from Treg cells, possibly due to a lack of Kyn. However, the precise mechanism of how Kyn is able to induce Foxp3 and inhibit IL-17 needs further investigation. Our findings underscore the potential for therapeutic targeting of the link between Ahr and kynurenines in the treatment of immune cell-mediated inflammatory diseases.
Materials and Methods
Mice.
C57BL/6J WT mice were obtained from CLEA. Ahr−/− males and Ahr−/+ females (C57BL/6J background) were provided by Yoshiaki Fujii-Kuriyama (University of Tsukuba, Tsukuba, Japan), kept and mated in our laboratory to generate Ahr−/− mice. To identify homozygous Ahr−/− mice, newborn mice were genotyped using PCRs performed with DNA obtained from the tails and specific primers. WT and Ahr−/− mice 6–8 wk old were used for these experiments. All mice were maintained under specific, pathogen-free conditions. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committees of the Graduate School of Frontier Biosciences, Osaka University.
BMDC Generation and Stimulation Conditions.
BMDC were generated from WT and Ahr−/− mice as follows. Briefly, bone marrow cells were flushed from tibiae and femurs of 6- to 8-wk-old C57BL/6J WT and Ahr−/− mice. The total bone marrow cells were counted (Fig. S5A). Then, 2 × 107 cells were seeded in 150-mm diameter cell culture dishes (Corning) in 30 mL RPMI medium 1640 (Sigma) supplemented with 10% FCS, 100 μg/mL streptomycin, 100 U/mL penicillin G, and 2.5 mM β-mercaptoethanol (complete RPMI medium 1640). On day 3, 30 mL complete RPMI medium 1640 containing 10 ng/mL GM-CSF (Peprotech) was added to the dishes. On day 6, 30 mL culture was removed and centrifuged. The pellet was resuspended in 30 mL fresh complete RPMI medium 1640 containing 10 ng/mL GM-CSF and returned to the same dish. On day 9, more than 80% of the nonadherent and loosely adherent cells expressed CD11c. BMDC were purified using a MACS column and CD11c MicroBeads (Miltenyi Biotec) and ≈90% of the purified BMDC were CD11b+ and CD11c+ (Fig. S5B). BMDC were seeded in 10 mL complete RPMI medium 1640 (5 × 106 cells/mL) and stimulated for 24 h with or without LPS (1 μg/mL; Sigma), phosphorothioate-modified CpG-ODN (CpG) (1 μM; Gene Design Inc.), and TCDD (160 nM; Cerilliant) as indicated. Stimulated BMDC were considered mature if they expressed high levels of MHC class II and CD86 (Fig. S5C).
qPCR.
Total RNA was prepared by RNeasy (Quiagen) according to the manufacturer's protocol. Reverse transcription of mRNA was performed in a thermal cycler (Applied Biosystems). The mouse Ahr probe (Gene Expression Assays: Mm00478930_m1, Applied Biosystems) and mouse IDO probe (Gene Expression Assays: Mm00492586_m1, Applied Biosystems) were used. For reference, we quantified mouse GAPDH (Applied Biosystems). The qPCR was carried out in an ABI PRISM 7900 HT (Applied Biosystems). Cycling conditions were 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. We applied the comparative ΔΔCt method normalized to GAPDH for IDO and Ahr mRNA quantitative analysis. The value of unstimulated cells was set at one and was used to calculate the fold change in stimulated cells.
DC–T-Cell Coculture.
Naive T cells were purified from the spleens of WT C57BL/6J mice using a CD4+ T-cell isolation kit and CD62L MicroBeads (Miltenyi Biotec). Purified naive T cells (CD4+CD62Lhi) (1 × 105 cells) were cocultured for 4 d with or without LPS- and CpG-stimulated WT or Ahr−/− BMDC (1 × 104 cells) in triplicate wells of a round-bottomed 96-well plate in a total volume of 200 μL/well. Naive T cells in the cocultures were stimulated with the Dynabeads Mouse T-activator CD3/CD28 (Invitrogen). L-Kyn (50 μM; Sigma) was added when indicated. After 4 d, IL-17 levels in the culture supernatants were measured using ELISAs (R&D Systems). T cells were stained with trypan blue, and the viability of the cells was assessed using an automated cell counter (Invitrogen).
Intracellular Cytokine and Foxp3 Staining.
After cocultured with LPS- or CpG-stimulated BMDC, T cells were stimulated with 50 ng/mL PMA (Calbiochem) and 800 ng/mL ionomycin (Calbiochem) for 5 h. GolgiStop (BD Pharmingen) was added for the last 2 h. Samples were then fixed and permeabilized with Cytofix/Cytoperm (BD Pharmingen). Cells were intracellularly stained with PE-conjugated anti–IL-17 antibodies (eBioscience) and FITC-conjugated anti–IFN-γ antibodies (eBioscience). For Foxp3 staining, T cells were fixed and permeabilized in Fixation/Permeabilization buffer (eBioscience) for 30 min at 4 °C before intracellular staining with FITC-antimouse Foxp3 antibodies (eBioscience). FACS was performed with a Cytomics FC500 system (Beckman Coulter).
Statistical Analysis.
Student t tests were used to analyze data for differences, and P values <0.05 were considered to be significant.
Supplementary Material
Acknowledgments
This work was supported by Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation and Chugai-Roche Pharmaceutical Co. Ltd (Tokyo, Japan).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014465107/-/DCSupplemental.
References
- 1.Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: A perspective on potential roles in the immune system. Immunology. 2009;127:299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ema M, et al. cDNA cloning and structure of mouse putative Ah receptor. Biochem Biophys Res Commun. 1992;184:246–253. doi: 10.1016/0006-291x(92)91185-s. [DOI] [PubMed] [Google Scholar]
- 3.Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci USA. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perdew GH. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem. 1988;263:13802–13805. [PubMed] [Google Scholar]
- 5.Ma Q, Whitlock JP., Jr A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 1997;272:8878–8884. [PubMed] [Google Scholar]
- 6.Kazlauskas A, Poellinger L, Pongratz I. Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (Aryl hydrocarbon) receptor. J Biol Chem. 1999;274:13519–13524. doi: 10.1074/jbc.274.19.13519. [DOI] [PubMed] [Google Scholar]
- 7.Schrenk D. Impact of dioxin-type induction of drug-metabolizing enzymes on the metabolism of endo- and xenobiotics. Biochem Pharmacol. 1998;55:1155–1162. doi: 10.1016/s0006-2952(97)00591-1. [DOI] [PubMed] [Google Scholar]
- 8.Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999;13:20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitot HC, Goldsworthy T, Campbell HA, Poland A. Quantitative evaluation of the promotion by 2,3,7,8-tetrachlorodibenzo-p-dioxin of hepatocarcinogenesis from diethylnitrosamine. Cancer Res. 1980;40:3616–3620. [PubMed] [Google Scholar]
- 10.Tomita S, et al. T cell-specific disruption of arylhydrocarbon receptor nuclear translocator (Arnt) gene causes resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced thymic involution. J Immunol. 2003;171:4113–4120. doi: 10.4049/jimmunol.171.8.4113. [DOI] [PubMed] [Google Scholar]
- 11.Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Cutting edge: Activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J Immunol. 2005;175:4184–4188. doi: 10.4049/jimmunol.175.7.4184. [DOI] [PubMed] [Google Scholar]
- 12.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 13.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 14.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura A, et al. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med. 2009;206:2027–2035. doi: 10.1084/jem.20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 17.Popov A, Schultze JL. IDO-expressing regulatory dendritic cells in cancer and chronic infection. J Mol Med. 2008;86:145–160. doi: 10.1007/s00109-007-0262-6. [DOI] [PubMed] [Google Scholar]
- 18.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 19.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81:247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 20.Mellor AL, Munn DH. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 21.Munn DH, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 22.Mellor AL, et al. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601–5605. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 23.Fujigaki H, et al. The signal transducer and activator of transcription 1α and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: Involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. 2006;139:655–662. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- 24.Wingender G, et al. Systemic application of CpG-rich DNA suppresses adaptive T cell immunity via induction of IDO. Eur J Immunol. 2006;36:12–20. doi: 10.1002/eji.200535602. [DOI] [PubMed] [Google Scholar]
- 25.Hill M, et al. IDO expands human CD4+CD25high regulatory T cells by promoting maturation of LPS-treated dendritic cells. Eur J Immunol. 2007;37:3054–3062. doi: 10.1002/eji.200636704. [DOI] [PubMed] [Google Scholar]
- 26.Edinger AL, Thompson CB. Antigen-presenting cells control T cell proliferation by regulating amino acid availability. Proc Natl Acad Sci USA. 2002;99:1107–1109. doi: 10.1073/pnas.042707999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munn DH, et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mellor AL, et al. Cutting edge: Induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol. 2003;171:1652–1655. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 29.Fallarino F, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor ζ-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 30.Vogel CFA, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jux B, Kadow S, Esser C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J Immunol. 2009;182:6709–6717. doi: 10.4049/jimmunol.0713344. [DOI] [PubMed] [Google Scholar]
- 32.Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Platten M, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 34.Criado G, Šimelyte E, Inglis JJ, Essex D, Williams RO. Indoleamine 2,3 dioxygenase-mediated tryptophan catabolism regulates accumulation of Th1/Th17 cells in the joint in collagen-induced arthritis. Arthritis Rheum. 2009;60:1342–1351. doi: 10.1002/art.24446. [DOI] [PubMed] [Google Scholar]
- 35.Jung ID, et al. Differential regulation of indoleamine 2,3-dioxygenase by lipopolysaccharide and interferon gamma in murine bone marrow derived dendritic cells. FEBS Lett. 2007;581:1449–1456. doi: 10.1016/j.febslet.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 36.Baban B, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fallarino F, et al. IDO mediates TLR9-driven protection from experimental autoimmune diabetes. J Immunol. 2009;183:6303–6312. doi: 10.4049/jimmunol.0901577. [DOI] [PubMed] [Google Scholar]
- 38.Hara T, et al. High-affinity uptake of kynurenine and nitric oxide-mediated inhibition of indoleamine 2,3-dioxygenase in bone marrow-derived myeloid dendritic cells. Immunol Lett. 2008;116:95–102. doi: 10.1016/j.imlet.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Belladonna ML, et al. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J Immunol. 2006;177:130–137. doi: 10.4049/jimmunol.177.1.130. [DOI] [PubMed] [Google Scholar]
- 40.Frumento G, et al. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall NB, Kerkvliet NI. Dioxin and immune regulation: Emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann N Y Acad Sci. 2010;1183:25–37. doi: 10.1111/j.1749-6632.2009.05125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 43.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: Much ado about IDO. Trends Immunol. 2003;24:242–248. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 44.Fallarino F, et al. Tryptophan catabolism generates autoimmune-preventive regulatory T cells. Transpl Immunol. 2006;17:58–60. doi: 10.1016/j.trim.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Marshall NB, Vorachek WR, Steppan LB, Mourich DV, Kerkvliet NI. Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol. 2008;181:2382–2391. doi: 10.4049/jimmunol.181.4.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mezrich JD, et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romani L, Zelante T, De Luca A, Fallarino F, Puccetti P. IL-17 and therapeutic kynurenines in pathogenic inflammation to fungi. J Immunol. 2008;180:5157–5162. doi: 10.4049/jimmunol.180.8.5157. [DOI] [PubMed] [Google Scholar]
- 48.Scott GN, et al. The immunoregulatory enzyme IDO paradoxically drives B cell-mediated autoimmunity. J Immunol. 2009;182:7509–7517. doi: 10.4049/jimmunol.0804328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heath-Pagliuso S, et al. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry. 1998;37:11508–11515. doi: 10.1021/bi980087p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.