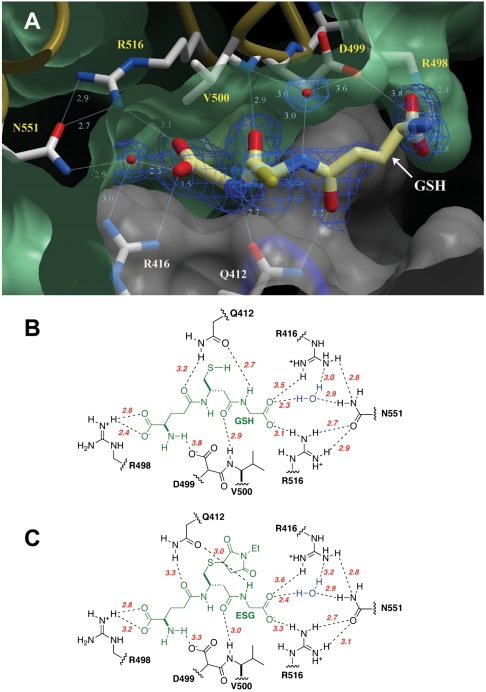
Fig. 1.
Glutathione binding to KefC. (A) GSH binds in a pocket formed at the interface of two KTN domains within a canonical dimeric assembly (green and white). Residues that coordinate glutathione include Q412 and R416 from one subunit, and R498-D499, the backbone amide of V500, as well as R516 and N551 indirectly through a water molecule, from the partnering fold. Electron density from a 2Fo-Fc map contoured at 1.5σ is shown for the ligand (blue wire). (B) Residues contributing to the coordination of GSH are shown with hydrogen bond lengths indicated in angstroms (red). (C) Equivalent schematic for the coordination of ESG by KefC.