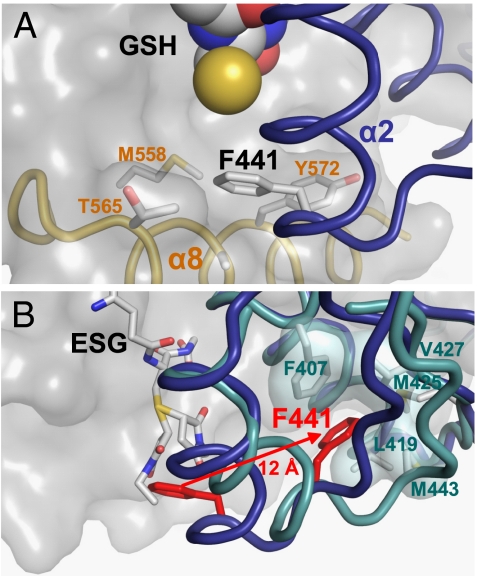
Fig. 3.
Mechanism of glutathione adduct modulation of KTN conformation. (A) The reduced thiol group of glutathione (yellow sphere) stabilizes the binding of a phenylalanine residue (F441) from α-helix 2 from one KTN subunit (blue) into a hydrophobic pocket formed by residues from α-helices 7 (M558) and 8 (T565; A569; Y572) from the partnering KTN subunit (gray surface). (B) When the thiol group of glutathione is conjugated to form an adduct, the attached chemical group (both diastereomers of N-ethylsuccinimido-S-glutathione shown here) displaces F441 (red) to an alternative conformation 12 Å distant, where it forms new interactions with hydrophobic residues within its own chain. The disruption of the original protein–protein interaction also leads to repositioning of α8, which is no longer observed in the crystallographic data.