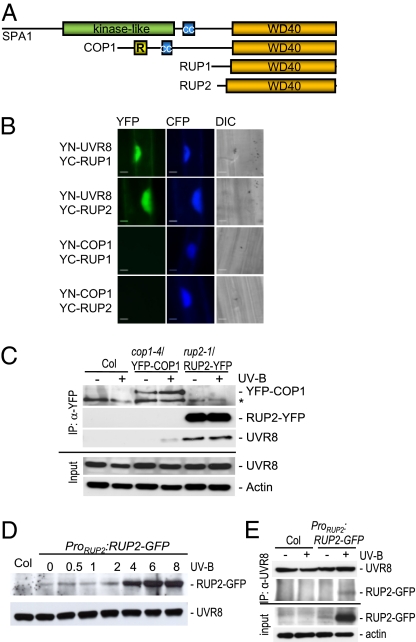
Fig. 2.
The RUP proteins interact directly with the UVR8 protein. (A) Schematic comparison of the protein domain structures of the three groups of WD40-repeat–containing repressors of photomorphogenesis (see also Fig. S3). (B) BiFC visualization of YC-RUP1 and YC-RUP2 interaction with YN-UVR8, but not with YN-COP1. A Pro35S:CFP control plasmid was always cobombarded to identify transformed cells before the analysis of YFP fluorescence. Specific CFP and YFP filter sets were used for microscopic analysis. Differential interference contrast images are shown. (Scale bar: 10 μm.) (C) Coimmunoprecipitation of endogenous UVR8 with RUP2-YFP. Coimmunoprecipitation of proteins using anti-YFP antibodies in extracts from rup2-1/Pro35S:RUP2-YFP transgenic seedlings. Four-day-old seedlings were irradiated with UV-B for 4 h (+UV-B) or mock-treated under a cutoff filtering out UV-B (−UV-B). An asterisk indicates a nonspecific cross-reacting band. (D) UV-B–responsive accumulation of RUP2-GFP protein expressed under its own promoter. Total protein was isolated from 4-d-old rup1 rup2/ProRUP2:RUP2-GFP transgenic seedlings that were irradiated with UV-B for the indicated times before harvesting. The protein gel blot was sequentially probed with anti-GFP and anti-UVR8 antibodies. (E) Coimmunoprecipitation of RUP2-GFP expressed under its own promoter with endogenous UVR8. Coimmunoprecipitation of proteins using anti-UVR8 antibodies in extracts from rup1 rup2/ProRUP2:RUP2-GFP transgenic seedlings and nontransgenic Col controls. Four-day-old seedlings were irradiated with UV-B for 6 h (+UV-B) or mock-treated under a cutoff filtering out UV-B (−UV-B).