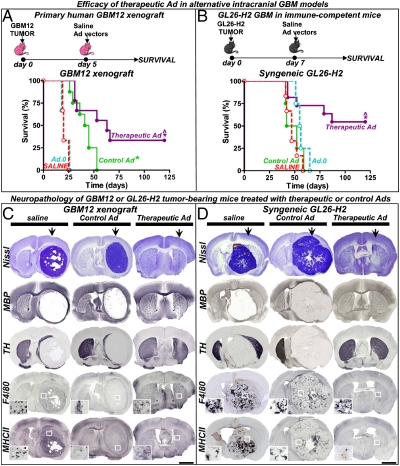
Fig. 5.
Efficacy and neurotoxicity of therapeutic Ads in alternative intracranial GBM models. (A) Rag1−/− mice were implanted with human primary GBM12 cells in the striatum and 5 d later they were treated with a single intratumoral injection of control (Ad.mhIL-4.TRE.mhIL-13) or therapeutic vector (Ad.mhIL-4.TRE.mhIL-13-PE), in combination with Ad.TetON. As controls they received saline or an empty Ad (Ad.0). (B) Immune-competent C57/B6 mice were intracranially implanted with GL26 cells expressing IL13Rα2 (GL26-H2) and treated 7 d later with the Ads. Animals were fed Dox chow. *P < 0.05 versus saline, ^P < 0.05 versus control Ad.mhIL-4.TRE.mhIL-13; Mantel log-rank test. (C and D) Neuropathology was assessed in moribund mice and long-term survivors (120 d) by Nissl staining and immunostaining of TH, MBP, F4/80 (macrophages/activated microglia), and major histocompatibility complex II (MHCII). The arrows indicate the injection site. Insets show higher-magnification microphotographs of the areas indicated with a white square. (Scale bars, 1,000 μm.) Note complete tumor regression in long-term survivors and the absence of neuropathological or inflammatory side effects.