Fig. 4.
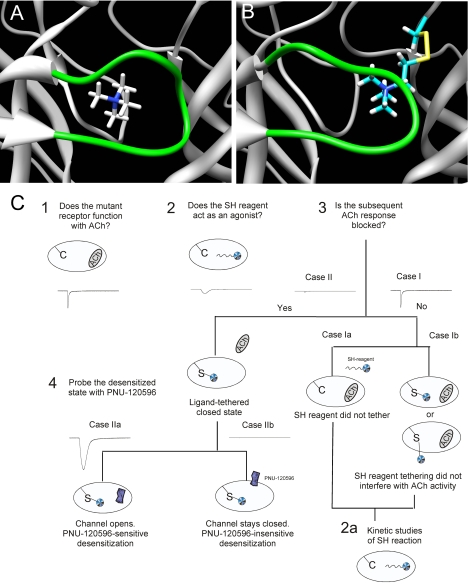
A, the docked pose of TMA in the LBD of α7. B, the predicted placement of the affinity ligand MTSET covalently bound to a cysteine at the location of Leu119. C, experimental design. Schematic of the design and hypotheses related to the possible events in the labeling study with the SH reagents, after the initial characterization of functional cysteine mutants. Case I represents the lack of functional evidence that a covalent bond formed between the cysteine mutant and the SH reagent. This could indicate two possible conditions: case Ia, in which no covalent modification occurred to because of unfavorable reaction conditions; and case Ib, in which the tethered α7 receptor can still accommodate ACh in one or more of the five binding sites, either the same domain where the SH reagent is attached or other free sites. Case II represents the condition in which the SH reagent has become covalently attached, and upon further application of ACh, activation of the ion channel is not observed, because of either SH reagent competitively occupying the agonist binding site or inducing ligand-bound nonconducting (i.e., desensitized) states. Receptors were subsequently challenged with the type II PAM PNU-120596 to determine whether the orientation of the reagent in the binding site was sufficiently agonist-like to induce desensitization that is reversible by PNU-120596 (case IIa) or whether the ligand is functionally indistinguishable from a tethered antagonist (case IIb).