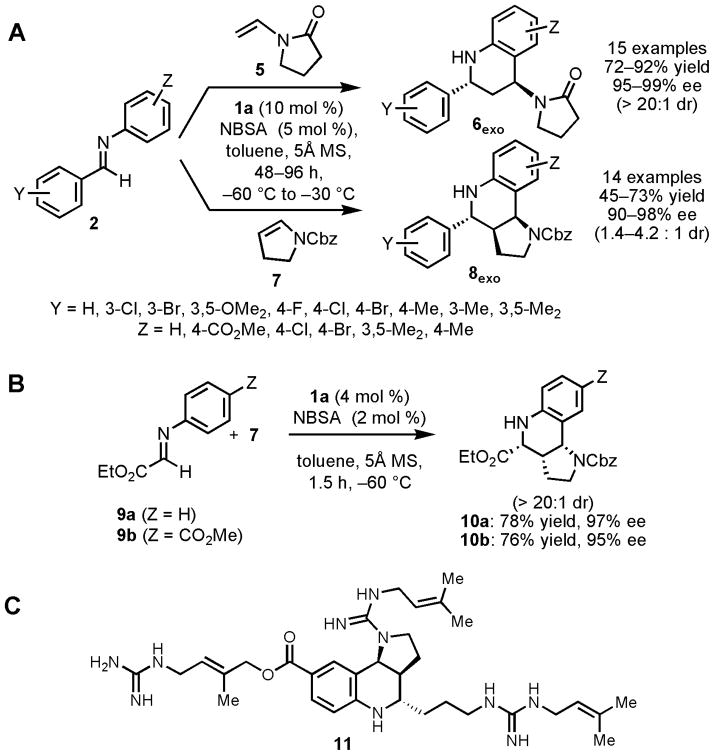
Figure 2.
(A, B) Asymmetric Povarov reactions catalyzed by 1a/NBSA with enamide 5 and enecarbamate 7 as the nucleophilic reacting partners. Molecular sieves (5Å) serve to sequester water introduced with the hygroscopic NSBA reagent, thereby preventing competing imine hydrolysis pathways. See Tables S2–S3 for yields and selectivities obtained with each substrate. (C) Martinelline (11), a natural product inhibitor of bradykinin B1 and B2 G protein-coupled receptors (IC50 = 6.4 and 0.25μM, respectively). A previous study demonstrated that a racemic form of ester 10b could be converted to (±)-11 after epimerization of the corresponding aldehyde, so the enantioselective synthesis of 10b described here constitutes a formal, enantioselective synthesis of martinelline (25).