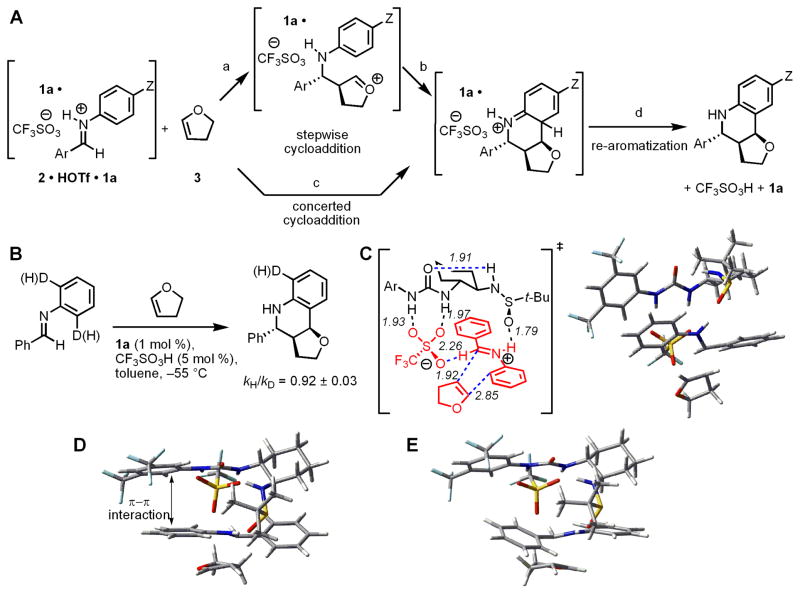
Figure 4.
(A) Possible mechanisms and rate-limiting steps in the asymmetric Povarov reaction. Z = an electron-donating or withdrawing substituent. (B) Kinetic isotope effect experiments to distinguish between different possible rate-limiting steps. (C) Geometry and energy-minimized lowest energy transition structure for cycloaddition calculated at the B3LYP/6-31G(d) level of density functional theory. Selected bond distances are shown in Å. Ar = 3,5-(CF3)2C6H3. (D) Alternate view of the structure in C highlighting the stabilizing π-π interaction between the aryl groups of the catalyst and the imimium ion undergoing cycloaddition. (E) Analogous view of the transition structure leading to the minor enantiomer of product. This transition structure lacks stabilizing π-π interactions, and is disfavored relative to the structure in D by 1.3 kcal/mol in calculations using the B3LYP/6-31G(d) method, and by 3.6–3.9 kcal/mol using M05-2X/6-31+G(d,p) or MP2/6-31G(d) single-point calculations. See Table S17 for further details.