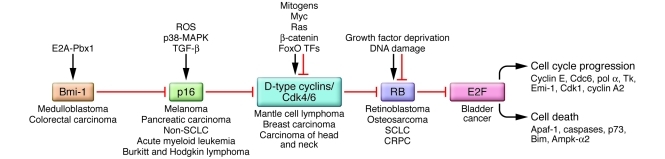
Figure 1. Inactivation of the RB pathway in human cancers.
Different components of the RB pathway are deregulated in human cancers, with the overall effect of derepressing E2F transcription factors that promote cell cycle progression or programmed cell death. When cell death pathways are inactivated, as is the case in most cancers, activation of E2Fs drives proliferation through induction of target genes such as cyclin E, CDC6, and CDK1. Loss of the RB1 tumor suppressor is prevalent in osteosarcoma and small-cell lung carcinoma (SCLC) in addition to its defining loss in human retinoblastoma, but it was initially confounding that its loss was not more commonly detected. However, with the discovery that p16/INK4A functions as an upstream inhibitor of CDK4 and CDK6 and thus as an activator of RB, and that loss of the p16/INK4A locus occurred in many of the cancers where RB1 loss was not detected, Weinberg and others proposed that functional inactivation of RB could be achieved in different tumors through targeting different components of the RB pathway (16). Thus, loss of p16/INK4A in melanoma, translocation of cyclin D1 in mantle cell lymphoma, or, indeed, overexpression of BMI-1 in medulloblastoma has been proposed to have similar functional readout to RB loss in terms of E2F activity and cellular proliferation. Ampk-α2, α2 subunit of AMPK; Apaf-1, apoptosis protease activating factor–1; E2A-Pbx1, the translocation product of fusion of the E2A gene to the Pbx1 gene; Emi-1, early mitotic inhibitor 1; FoxO TFs, forkhead box O subclass of transcription factors; Tk, thymidine kinase; pol α, DNA polymerase-α.