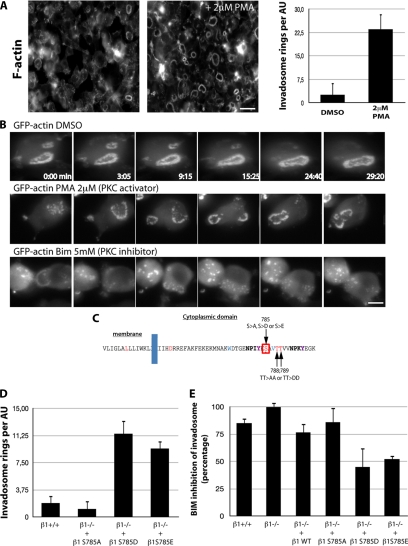
Figure 4.
PKC regulates invadosome autoassembly by phosphorylating Ser785 of β1A integrins. (A) PKC activation by a 60-min treatment of pOBL-SrcYF cells with 2 μM PMA induces a massive increase in invadosome rosette autoassembly, visualized by phalloidin staining. (B) Extracted images from time series (in minutes) from representative observations of pOBL-SrcYF cells expressing GFP-actin and treated with either dimethyl sulfoxide (DMSO; control), the PKC activator PMA (2 μM), and the PKC inhibitor BIM (5 mM). PKC activity regulates the dynamics and maintenance of the invadosome autoassembly state. (C) Amino-acyl sequence of the cytoplasmic domain of β1A integrin and the location of the main PKC targets. (D) Quantification of invadosomes per Airy unit (AU) shows that a mutation mimicking a constitutive phosphorylated form of Ser785 (Asp or Glu) dramatically increases the formation of rosettes in pOBL-SrcYF β1−/− cells. The nonphosphorylatable mutant of β1 at this site (S785A) has no effect on rosette formation. (E) The number of rosettes/AU was quantified in pOBL-SrcYF cells treated with 2 μM PMA or simultaneously with PMA and 5 mM BIM for 60 min. The inhibitory effect of BIM was determined by calculating the percent inhibition of invadosome rosette formation. Mutants mimicking a constitutively phosphorylated Ser785 (S785D and S785E) show only 50% inhibition after BIM treatment, indicating that this residue on β1 is a major target of PKC in the regulation of invadosome autoassembly. Bars, 20 μm (A) and 5 μm (B).