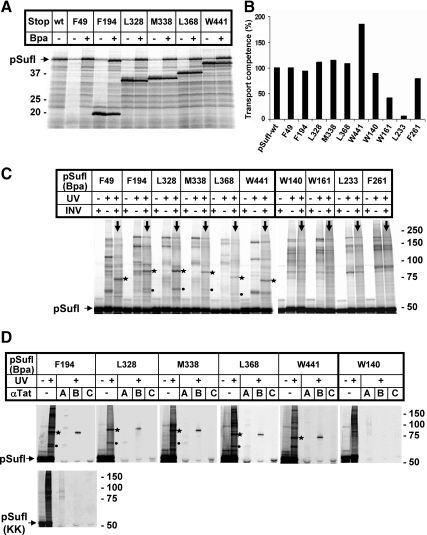
Figure 2.
On membrane-targeting, the surface of pSufI is found predominantly in the vicinity of TatB. (A) Codons of the sufI DNA that specify the indicated amino acids were mutated to amber stop codons. The resulting DNAs were transcribed and translated in vitro by an E. coli cell-free extract prepared from a strain that expresses an amber suppressor tRNA and the cognate Bpa-specific tRNA-synthetase. Radiolabeled translation products were separated by SDS-PAGE and are visualized by Phosphorimaging. The electrophoretic mobilities of molecular mass standards are indicated on the left. In contrast to the approx. 50-kDa wild-type (wt) precursor of SufI (pSuf), smaller translation products were predominantly expressed from the amber mutant DNAs when Bpa was missing. These smaller translation products match the molecular masses expected for premature termination at the individual amber codons. The shortest fragment of 48 amino acids was not retained by the polyacrylamide gel used here. In addition to each premature termination product, some full-size pSufI was synthesized in the absence of Bpa, obviously resulting from read-through of the mutant mRNAs. Addition of Bpa led to a clear increase in full-size pSufI of each variant, indicating suppression of the amber codons by the incorporation of the cross-linker Bpa. (B) Wild-type pSufI and its indicated Bpa variants were synthesized in vitro in the presence of inside-out inner membrane vesicles, and the transport efficiency of each mutant was analyzed as described in Materials and Methods. Transport efficiency of wt-pSufI was set 100%. (C) After synthesis of each indicated Bpa variant of pSufI in the presence or absence of membrane vesicles (INV), samples were irradiated with UV light or mock-incubated before SDS-PAGE and Phosphorimaging. UV irradiation led to numerous radiolabeled bands larger in size than pSufI, only few of which were specifically obtained in the presence of INV (lanes marked with downward pointing arrows). The most prominent of those photo-adducts are labeled with asterisks and dots. Of notice, no membrane-specific adducts were obtained for SufI variants W140, W161, L233, F261 carrying Bpa in the interior of the folded structure. (D) The indicated Bpa variants of pSufI were synthesized in vitro in the presence of INVs. Samples irradiated with UV light were either directly prepared for SDS-PAGE or only after coimmunoprecipitation with antibodies directed against TatA, TatB, and TatC (αTat). Asterisk-labeled adducts are recognized by anti-TatB antibodies and dot-labeled ones by anti-TatA antibodies. No Tat-specific adduct was observed for the internal W140 variant of pSufI, nor were any specific cross-links obtained with the nonfunctional KK-precursor of SufI.