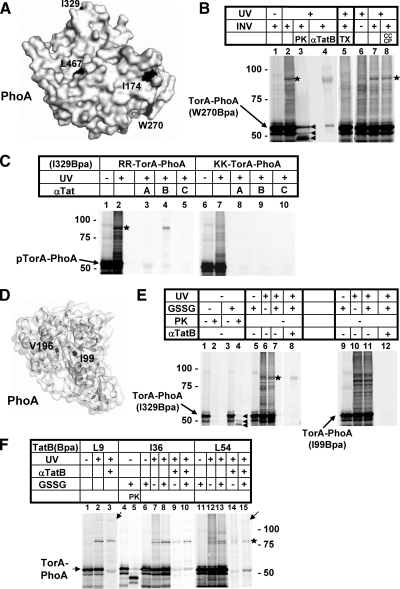
Figure 5.
TorAPhoA shows the same interaction with TatB as pSufI. (A) Surface-exposed residues I174, W270, I329, and L467 of PhoA used for the incorporation of the cross-linker Bpa. Structural data were taken from Wang et al., (2005). (B) The 55 kDa Tat precursor protein TorA-PhoA carrying Bpa at the surface position W270 (arrow) was synthesized in vitro with INVs added as indicated. When irradiated with UV-light, a major photo-cross-link (asterisk) was obtained that was recognized by anti-TatB antibodies (αTatB) and was absent from reactions devoid of membranes (lane 6). It was completely digested by Proteinase K (PK) (lane 3) in contrast to the PK-resistant translocated TorA-PhoA species (arrowheads) and therefore not membrane-protected. The three translocated species of TorA-PhoA from top to bottom are precursor, mature form and a translocation-arrested species (Panahandeh et al., 2008). Solubilization of the INVs' Tat machinery by 1% Triton X-100 prevented formation of the TatB adduct of TorA-PhoA (lane 5), whereas dissipation of the PMF by CCCP did not (lane 8). (C) TorA-PhoA (arrow) carrying Bpa at the surface position I329 was synthesized in vitro in the presence of INVs, irradiated with UV-light, and immunoprecipitated with antisera against TatA, TatB, and TatC (lanes 1–5). The only Tat-specific photo-adduct obtained was the one recognized by anti-TatB antibodies (asterisk). Cross-linking was completely abolished if the signal sequence of TorA-PhoA carried a KK-mutation of the consensus RR-motif (lanes 6–10). (D) Internal residues I99 and V196 of PhoA used for the incorporation of Bpa. (E) Comparison between the surface-exposed Bpa variant I329 of TorA-PhoA and the internal variant I99. Lanes 1–4 illustrate that transport of TorA-PhoA into INV proceeded only when TorA-PhoA was synthesized under oxidizing conditions, which were achieved by the addition of oxidized glutathione (GSSG). Transport is indicated in lane 4 by the appearance of the PK-resistant TorA-PhoA species specified in B (arrowheads). In contrast to the internal Bpa variant I99, the surface-exposed variant I329 formed adducts with TatB (asterisk) both under reducing and oxidizing conditions. (F) Cross-linking of the transmembrane and amphipathic helix of TatB to TorA-PhoA. The two TatB variants L9Bpa and L54Bpa again yielded higher molecular mass adducts (arrows). Cross-linking was observed both under reducing and oxidizing conditions.