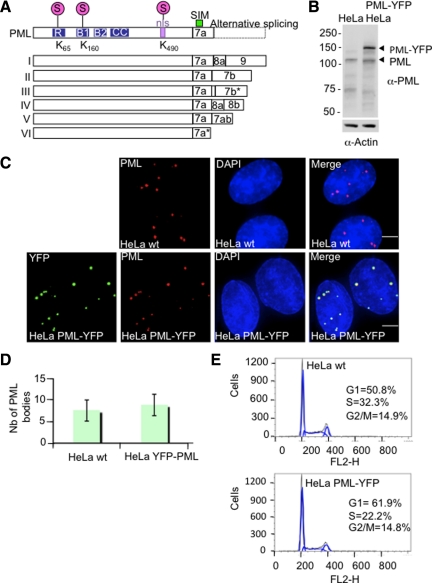
Figure 1.
Establishment and characterization of HeLa PML-YFP stable cell line. (A) Schematic showing the nuclear isoforms of PML that share the same N-terminal region but differ in their C termini due to the alternative splicing of exons 7–9. Nuclear PML isoforms I to V harbor three SUMOylation sites (K65, K160, and K490), a SUMO-interacting motif (SIM), a nuclear localization signal (nls), and a RBCC motif containing a RING finger domain (R) adjacent to two zinc coordinating B-boxes and a coiled-coil domain (CC). PML VI differs from the other PML isoforms due the absence of a SIM domain. (B) Whole cell extracts from parental HeLa cells and HeLa PML-YFP were analyzed by SDS-PAGE followed by Western Blot using a rabbit anti-PML antibody. An anti-actin antibody was used as a control of loading. (C) HeLa were stably transfected with a plasmid expressing PML-YFP placed under the control of EF1 promoter. Endogenous PML in parental HeLa cells (wt) was detected with a chicken antibody to PML and PML in cells stably expressing the PML-YFP fusion protein was detected by immunofluorescence using a mouse anti-PML antibody whereas expression of the fluorescent-tagged PML protein was visualized by YFP fluoresence. DNA was stained with DAPI. Bar, 5 μm. (D). Graph showing the number of PML bodies in HeLa wt and HeLa PML-YFP. (E) Cell cycle analysis of HeLa and HeLa PML-YFP cell lines was determined by flow cytometry. The percentage of cells in G1, S and G2/M phase was determined by measuring the intensity of DNA staining with propidium iodide (FL2-H).