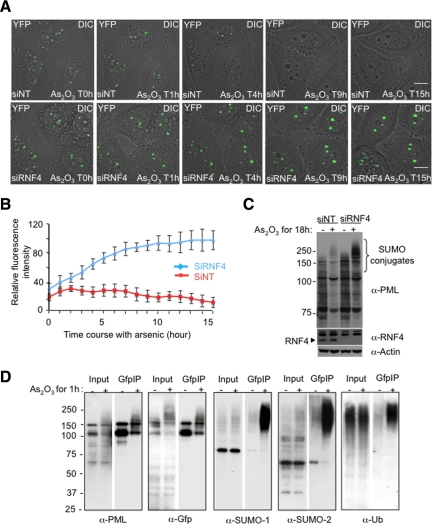
Figure 2.
Effect of RNF4 on arsenic-induced PML degradation in real time. (A) Time lapse experiments were performed on HeLa PML-YFP stable cells transfected with a nontarget siRNA (siNT) or a siRNA against RNF4 (siRNF4) and exposed to 1 μM arsenic for 18h. PML-YFP was imaged in real time by fluoresence microscopy over 15 h by collecting a stack of 20 sections with the YFP channel (green) and one image with the differential interference contrast (DIC) every 15 min. The projected z-sections collected in YFP channel were merged to the respective DIC image to monitor the position of PML NB within the cells. Bar, 5 μM. (B) Fluorescence intensity of PML bodies was quantified by defining a region of interest containing one PML body and comparing it a region in the nucleoplasm. Relative fluorescence intensity represents the difference of intensities between these two regions. The graph shows mean values from at least 10 cells. (C) Whole cell extracts from PML-YFP HeLa cells transfected with siRNA to RNF4 or a nontarget siRNA were analyzed by SDS PAGE followed by Western Blotting with a chicken anti-PML antibody to show the accumulation of PML in the absence of RNF4. Depletion of RNF4 was controlled with a rabbit anti-RNF4 antibody. (D) Nuclear extracts from PML-YFP HeLa cells either untreated or exposed to 1 uM arsenic for 1 h were incubated with GFP-trap beads and bound proteins collected. Proteins were eluted from the beads and analyzed by SDS-PAGE followed by Western blotting with antibodies against PML, GFP, SUMO-1, SUMO-2, and ubiquitin to evaluate proteins bound to PML-YFP. The input represents 10% of the nuclear lysate.