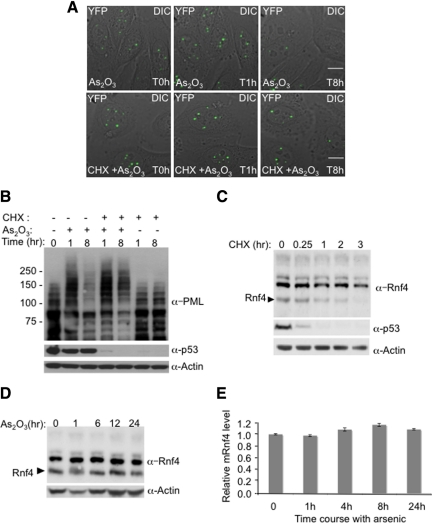
Figure 4.
Inhibition of RNF4 synthesis blocks arsenic-induced PML degradation. (A and B) Arsenic fails to induce the degradation of PML when protein translation is inhibited. HeLa PML-YFP stable cells were incubated with 1 μM arsenic and 50 μg/ml cycloheximide and analyzed by fluorescence microscopy for 16h (A). A stack of 20 z-sections was collected every 15 min in the YFP channel (green), and one image was taken with differential interference contrast (DIC). The projected z-stacks were merged to the respective DIC image. Bar, 5 μM. The level of PML and p53 proteins were analyzed during arsenic and cycloheximide treatment by SDS PAGE followed by Western Blotting with chicken PML and mouse p53 antibodies (B). (C) Monitoring of RNF4 and p53 protein levels during cycloheximide treatment. PML-YFP HeLa cells were incubated with 50 μg/ml cycloheximide, and whole cell extracts were analyzed at different times by SDS PAGE followed by Western Blotting with rabbit RNF4 and mouse p53 antibodies. (D) Arsenic does not alter the level of RNF4 protein. Total cells extracts from HeLa PML-YFP exposed to arsenic for 24 h were analyzed by SDS PAGE followed by Western blot with a rabbit anti-RNF4 antibody to follow the level of RNF4 during arsenic treatement. (E) Quantification of Rnf4 transcripts during arsenic treatment. Total RNA from HeLa PML-YFP stable cells exposed to 1 μM arsenic for 24 h was extracted and real-time quantitative PCR was performed with specific gene primers for RNF4 and actin. The graph shows the relative mRNF4 level after normalization to actin transcripts.