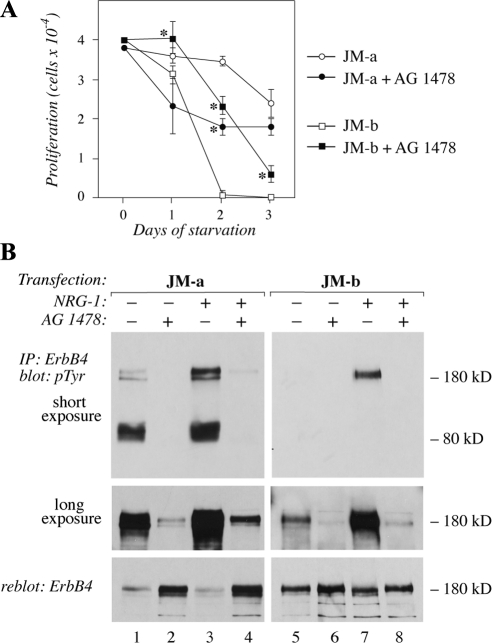
Figure 5.
The effect of inhibition of ErbB tyrosine kinase activity on cells expressing ErbB4 isoforms. (A) NR6 cells expressing JM-a CYT-2 or JM-b CYT-2 were plated onto 24-well plates in the presence of 10% FCS. The next day the medium was replaced with serum-free medium containing 0 or 10 μM of the ErbB kinase inhibitor AG 1478. Adherent cells were counted at indicated time points with hemocytometer. *p < 0.05 for a difference between AG 1478-treated and control cells. (B) Cells were starved overnight without serum, treated for 6 h with 0 or 10 μM AG 1478, and stimulated for 15 min with 0 or 50 ng/ml NRG-1. ErbB4 tyrosine phosphorylation was analyzed by immunoprecipitation with an anti-ErbB4 antibody followed by Western blotting with an anti-phosphotyrosine antibody. Two different exposures of the phosphotyrosine blot are shown. Membrane was reblotted with an anti-ErbB4 antibody. Binding of the anti-phosphotyrosine antibody 4G10 masks the epitope for the anti-ErbB4 sc-283, resulting in an apparently reduced ErbB4 signal for the heavily phosphorylated ErbB4 species in the reblot (lanes 1 and 3).