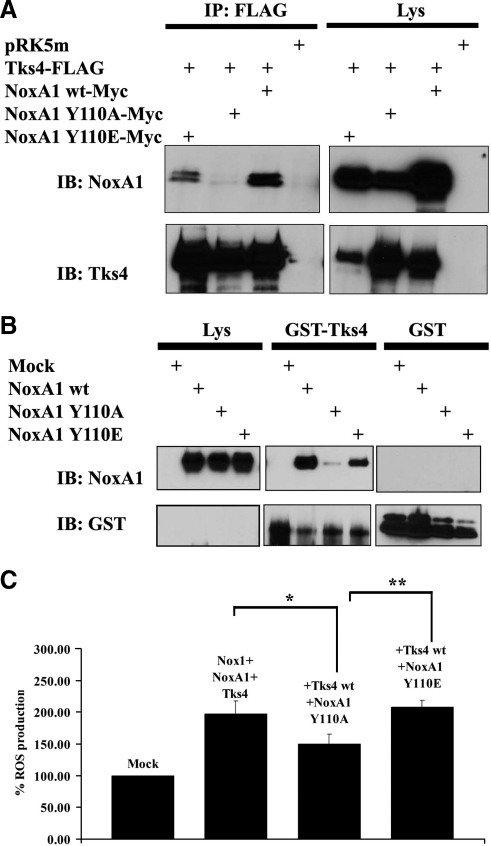
Figure 3.
The abolishment of NoxA1 phosphorylation on Tyr110 blocks its binding to Tks4 and decreases Nox1-dependent ROS generation. (A and B) The integrity of Tyr110 of NoxA1 is necessary for its binding to Tks4. One representative experiment from three separate experiments is shown. (A) Coimmunoprecipitation analysis was performed in HEK293 cells transfected as indicated with Flag-tagged Tks4 and Myc-tagged wild type NoxA1, unphosphorylable NoxA1 Y110A, and phosphomimetic NoxA1 Y110E. After 24 h, cells were lysed and immunoprecipitation (IP) was carried out using Flag antibody. The interaction between NoxA1 and Tks4 was tested by immunoblot (IB) using NoxA1 antibody (upper section), while comparable expression levels of transfected Flag-tagged Tks4 in cell lysates and immunoprecipitation efficiency was assessed by reblotting the membrane with Tks4 specific antibody (lower section). (B) HEK293 cells were transfected as indicated with empty vector or with wild-type NoxA1, unphosphorylable NoxA1 Y110A, and phosphomimetic NoxA1 Y110E. After 24 h, cells were lysed and cell lysates were used as the source of different NoxA1 proteins and incubated as indicated with equal amounts of GST alone or GST-fusion Tks4 protein, which were prebound to glutathione-Sepharose beads. GST pulldown was performed as described in Material and methods. The interaction between GST-fusion Tks4 and different NoxA1 proteins and comparable levels of NoxA1 proteins in cell lysates was tested using the NoxA1 antibody (upper panel). The reblot using GST-specific antibody in the lower panel indicates that GST-fusion protein were present at similar levels in the GST pulldown analysis. (C) The abolishment of NoxA1 phosphorylation on Tyr110 decreases Tks4-mediated Nox1-dependent ROS generation. ROS generation was monitored in HEK293 cells transfected as indicated using luminol-based chemiluminescence (CL) assay. One representative experiment from three separate experiments is shown, and data are given as mean of triplicates ± SD. *p < 0.008; **p < 0.01.