Abstract
The nervus terminalis (NT) is a vertebrate cranial nerve whose function in adults is unknown. In bonnethead sharks the nerve is anatomically independent of the olfactory system, with two major cell populations within one or more ganglia along its exposed length. Most cells are immunoreactive for either gonadotropin-releasing hormone (GnRH) or RFamide-like peptides. To define further the cell populations and connectivity, we used double-label immuno-cytochemistry with antisera to different isoforms of GnRH and to choline acetyltransferase (ChAT). The labeling patterns of two GnRH antisera revealed different populations of GnRH immunoreactive (ir) cell-profiles in the NT ganglion. One antiserum labeled a large group of cells and fibers, which likely contain mammalian GnRH (GnRH-I) as described in previous studies, and which were ChAT immunoreactive. The other antiserum labeled large club-like structures, which were anuclear, and a sparse number of fibers, but with no clear labeling of cell bodies in the ganglion. These club structures were choline acetyltrasferase (ChAT) negative, and preabsorption control tests suggest they may contain chicken-GnRH-II (GnRH-II) or dogfish GnRH. The second major NT ganglion cell-type was immunoreactive for RF-amides, which regulate GnRH release in other vertebrates, and may provide an intraganglionic influence on GnRH release. The immunocytochemical and anatomical differences between the two GnRH immunoreactive profile types indicate possible functional differences for these isoforms in the NT. The club-like structures may be sites of GnRH release into the general circulation since these structures were observed near blood vessels and resembled structures seen in the median eminence of rats.
Keywords: Nervus terminalis, terminal nerve, elasmobranch, gonadotropin releasing hormone, GnRH-I, GnRH-II, RF-amide, FMRFamide, LPLRFamide
1. INTRODUCTION
The nervus terminalis (NT), which is found in all vertebrate groups, is the most rostral cranial nerve. Although its function has yet to be determined, cells and fibers within the NT contain the hormone gonadotropin-releasing hormone (GnRH; Phillips et al., 1987; Schwanzel-Fukuda and Silverman, 1980; Munz et al., 1982; Wirsig and Getchell, 1986; Wirsig and Leonard, 1986b; White and Meredith, 1995; Kim et al., 1999). In elasmobranchs, the NT contains a higher concentration of GnRH than any other part of the forebrain (Nozaki, 1984; Demski et al., 1987), suggesting NT may be important for the reproductive function of GnRH.
The central trunk of the NT extends as a bundle of GnRH-positive and -negative fibers from the peripheral NT ganglion to ventral-caudal regions of the telencephalon, towards the hypothalamus (Stell, 1984; Wright and Demski, 1991; Lovejoy et al., 1992a; Oka and Matsushima, 1993; Forlano et al., 2000). There is no pituitary portal system in elasmobranchs (Dodd, 1983) and it is unknown where GnRH is released or how it reaches gonadotropes in the ventral lobe of the pituitary. It has been proposed that some of the NT fibers lead to the ventral telencephalon or septal area where they might release GnRH (Nozaki, 1985; Lovejoy et al., 1992a). Numerous GnRH immunoreactive (ir) fibers also extend throughout the forebrain suggesting a neuromodulatory action on other neural systems (Pfaff et al., 1987; Oka and Matsushima; 1993, Millar, 2003). In Atlantic stingrays, stimulation of the peripheral trunk of NT led to an increase in measurable levels of GnRH in the cerebrospinal fluid, presumably by synaptic release from NT fibers in the brain (Moeller and Meredith, 1998).
The NT has also been suspected of having a chemosensory component since it connects the nose and the brain, and fibers from the peripheral trunks extend into the nasal epithelium (Demski and Northcutt, 1983; Wirsig and Leonard, 1986a; Demski et al., 1987; Koza and Wirsig-Wiechmann, 2001). However, an intrinsic chemosensory function has not been demonstrated and NT fibers appear not to influence olfactory sensory signals directly, at least in elasmobranchs (Meredith and White, 1987). GnRH itself does modulate some olfactory responses in axolotls (Park and Eisthen, 2003) and mud-puppies (Zhang and Delay 2007). NT is present in mammals and, in male golden hamsters, NT damage caudal to the olfactory bulb leads to some deficits in mating behavior (Wirsig and Leonard, 1987; Wirsig-Wiechmann, 1997). A response generally considered to be GnRH dependent, the testosterone secretion induced by odors from female hamsters in estrus, was not decreased by NT lesions (Wirsig-Wiechmann, 1993), although a NT-dependent pathway might have survived, via NT connections to the accessory olfactory bulb (Wirsig and Leonard 1986a) and on to medial amygdala.
It is now understood that GnRH decapeptides are produced from three genes (in fish) and can be classified into three corresponding families (GnRH I, II and III) (Fernald and White 99, Millar et al 04). Differences in anatomical location suggest functional differences in the release and action of different GnRH isoforms (Phillips et al 1987, Muske, 1993; Sherwood et al 1993, Muske et al 1994, Rissman et al 1995, King and Miller, 1995; Latimer et al 2000, Millar, 2003; Temple et al., 2003, Millar 2004). The putative ancestral form, GnRH-II (His5Trp7Tyr8-GnRH; formerly chicken-GnRH-II; Millar 2003), generally expressed in a cluster of cells in the midbrain, is completely conserved across species. It can act as a neuromodulator (Troskie et al 1997), and can facilitate reproductive behavior in birds (Maney et al 1997) and food-restricted musk shrews (Temple et al 2003). At least one additional isoform is expressed elsewhere in the brain (Millar et al 2004), generally a GnRH-I or GnRH-III-family peptide responsible for LH release from the pituitary. GnRH-I peptides are quite variable in structure and are most visible in a separate population of cells along the NT-septum-preoptic axis (Jennes and Stumpf, 1986; Peter et al, 1987; Lehman et al., 1987; Silverman et al, 1987; Forlano et al., 2000; Dubois et al., 2002). The originally identified peptide, mammalian GnRH-I (mGnRH) is the releasing peptide in most mammals, amphibia and probably in some teleost fish (Dubois et al 02). A variant GnRH-I (Gln8-GnRH; formerly chicken-I GnRH) is the releasing peptide in birds and reptiles, and other variants have been identified in the preoptic area of several teleosts and one mammal (Grove-Strawser et al 2002). Most teleosts have the (well conserved) GnRH-III peptide (Trp7-Leu8-GnRH, originally named salmon GnRH) replacing GnRH-I as the LH-releasing peptide (Pallevich et al 09) but some also express additional isoforms (Pandolfi et al 05). Mammalian GnRH-I (mGnRH) can also act as a neuromodulator if applied exogenously (Pan et al 88) and has been suggested to mediate a neural (intracerebral) facilitation of reproductive behavior in male and female mammals (Dorsa and Smith 1980, Moss and Dudley 1989; Fernandez-Fewell and Meredith 1995, Blake and Meredith 2010) independently of its LH releasing function.
Elasmobranchs, which lack a developed pituitary portal system, express GnRH II in the midbrain, and often two or more other isoforms, concentrated more in neurons of the NT nerve and ganglia than in the preoptic area, and generally considered to be involved in gonadotropin release.
Within the NT, a close relationship has been shown in elasmobranchs and some other species, between cells expressing GnRH and fibers immunoreactive to antisera raised against RF-amide (RFa) peptides; e.g. FMRFamide (Phe-Met-Arg-Phe-NH2; Stell, 1984; Muske and Moore, 1988; Wirsig-Wiechmann and Basinger, 1988; Chiba, 2000) or LPLRFamide (Leu-Pro-Leu-Arg-Phe-NH2; White and Meredith 1995).
A similar close relationship has recently been demonstrated in the ventral forebrain of birds and mammals (see Tsutsui et al 2010, Johnson et al 2007, Simonneaux et al 2008). The RF-amide family peptides, including kisspeptin, gonadotropin inhibitory peptide (GnIH) and RFRP1/3, are critically involved in regulating release of GnRH acutely and according to season in birds, mammals and reptiles and probably in teleosts (Johnson et al 2007, Simonneaux et al 2008, Clarke et al 2009, Oka et al 09). In elasmobranchs, the function of the RFa-ir cell groups in NT is unknown but they are placed in an ideal location to influence GnRH release from the GnRH system(s) in NT.
In bonnethead sharks (Sphyrna tiburo) one or more ganglia are situated along the exposed length of the NT nerve between the olfactory bulbs and the nerve’s entry into the forebrain. Cells within the large main ganglion can be classified into two types. One cell class is GnRH-ir as described above and is co-localized with a plexus of catecholamine-ir fibers. A second, distinct class is immunoreactive to antibodies raised against RFa peptides (including LPRFa). Both were acetylcholinesterase positive (White and Meredith 1995).
Here we report on two classes of GnRH-ir cell-profiles in the NT ganglion in addition to the RF-amide-ir cells. They express different isoforms of GnRH and one (only) shows co-localization of choline acetyltransferase (ChAT) immunoreactivity. Their anatomical and cytochemical differences suggest potential differences in function. Early parts of this study were published in abstract form (Moeller et al., 1997).
2. RESULTS
The GF-5 antiserum, raised against salmon GnRH, labeled large clusters of cells distributed throughout the NT ganglion (Figs. 1A, 3A). Cells tended to be oval and near 20 μm in diameter. The antiserum also labeled bundles of fibers, some of which can be seen traveling through both the peripheral and central nerve trunks. Surrounding the labeled cells were areas, generally similar in size, with no labeled cell bodies. Tracts of labeled fibers, however, traversed clusters of these unlabeled cell bodies.
Figure 1.
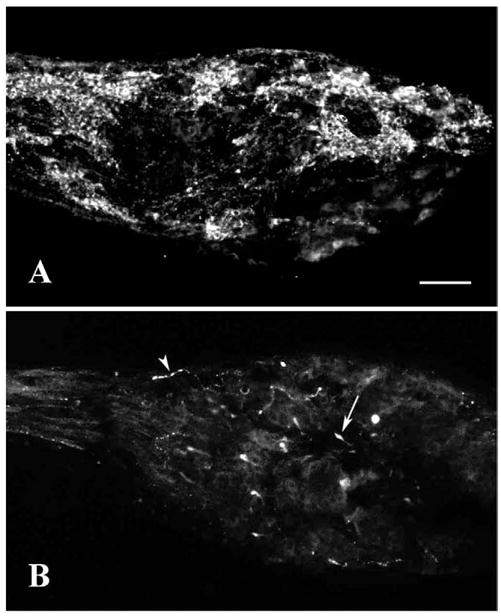
Examples of differences in GnRH immunocytochemistry in the ganglion of bonnethead NT. A: Labeling with GF-5 antiserum showing large clusters of GnRH-ir cells and tracts of fibers. B: Labeling with 7CR-10 antiserum showing relatively sparse labeling of varicose fibers (arrowhead) and a few club-like structures (arrow). Scale bar = 100 μm.
Figure 3.
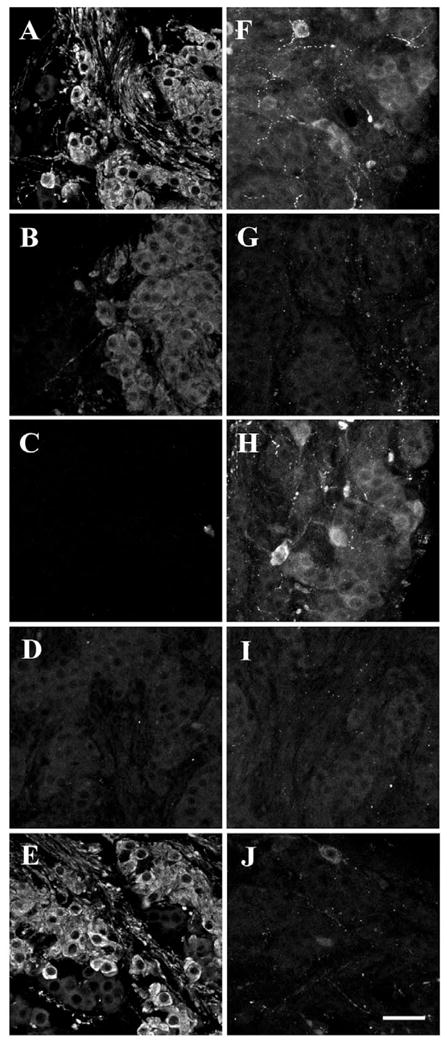
Examples of control studies using different isoforms of the GnRH peptide to block labeling with the GF-5 (B-E) and 7CR-10 antisera (G-J) compared to the normal labeling patterns of GF-5 (A) and 7CR-10 (F). Control sections were preabsorbed with either chicken-II (B & G), mammalian (C & H), salmon (D & I), or lamprey GnRH (E & J). Scale bar = 50 μm.
The 7CR-10 antiserum, raised against dogfish GnRH (His5,Trp7,Tyr8 GnRH), showed a strikingly different labeling pattern (Figs. 1B, 3F). Compared to the GF-5 pattern, overall labeling was extremely sparse. Cell bodies were rarely labeled: only two ganglia out of twelve had one or two faintly stained structures, which appeared cell-like (Fig. 3F). A few isolated fibers with varicosities traversed all segments of the ganglion in no apparent overall pattern. Occasionally larger club-like structures capped the ends of these isolated fibers. Some were up to 20 μm in diameter, the size of cell bodies. In sections of the NT ganglion double labeled with 7CR-10 antiserum and Hoechst dye, there was clearly no co-localization of the blue fluorescent nuclear marker with the large club-like structures labeled with this GnRH antiserum (Fig. 2A). Moreover, these club-like structures were often observed near blood vessels. With the confocal microscope it was possible to image blood vessels with DIC transmission optics and then take a single 1 μm optical section of fluorescence at the same focal plane and position. The micrograph in Figure 2B, for example, shows a blood vessel traversing the section surrounded by cell structures labeled with the 7CR-10 antiserum. A club-like structure (arrow) can be seen near the cross-section of a blood vessel (arrowhead), as it runs perpendicular to the plane of the section. Numerous other club-like structures were also observed adjacent to blood vessels. In fact, whenever these structures were investigated by confocal sectioning they were located next to blood vessels. This examination included one or two clubs in at least four ganglia acquired from separate specimens. However, a quantitative and systematic study of this relationship was not undertaken here.
Figure 2.
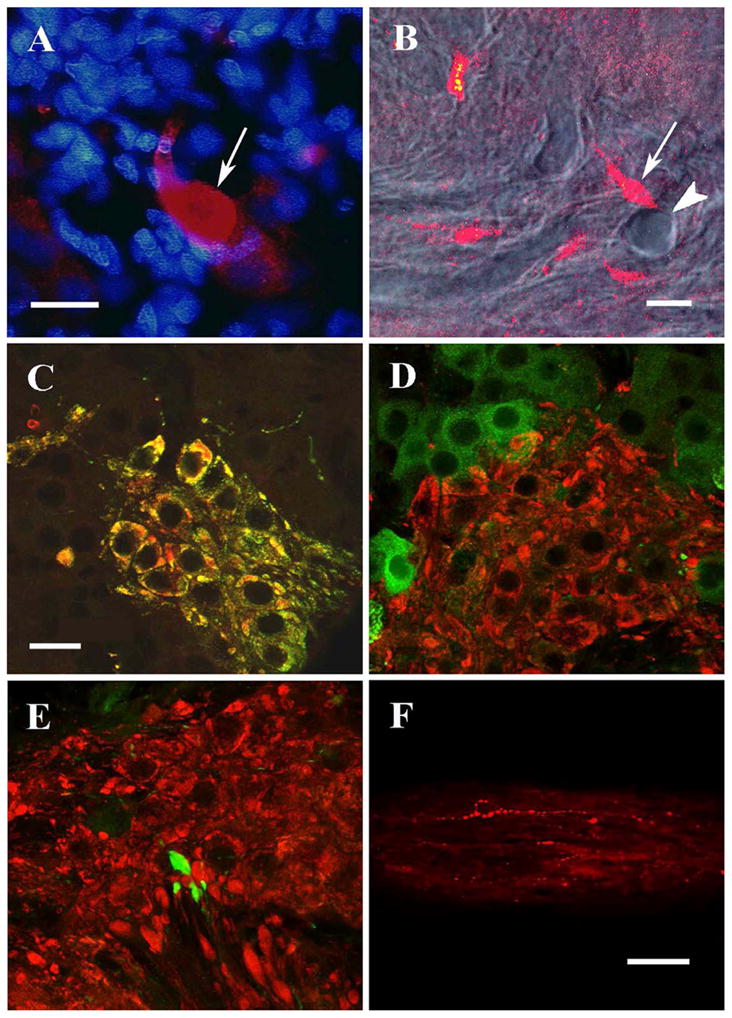
Confocal micrographs of GnRH and ChAT immunocytochemistry in NT ganglion. A: Large club-like structure labeled with 7CR-10 (arrow) contains no blue-stained nuclear material (Hoechst dye). B: Combined DIC/single-confocal slice image: A club-like structure (arrow) is adjacent to a blood vessel (arrowhead) that traverses the plane of section, exiting the section near the club. C: A projected stack of confocal images showing ChAT-ir (red) and GnRH/GF-5-ir (green) are co-localized resulting in clusters of cells labeled yellow. All single confocal images also showed colocalization in all cells. D: FMFRa-ir (green) and ChAT-ir (red) are not co-localized. E: Club-like structures labeled with 7CR-10 (green) are not co-localized with ChAT-ir (red). Small areas of yellow in D and E are an artifact of stacked confocal images from adjacent optical sections superimposed on one another, and thus do not indicate co-localization. F: Central nerve trunk labeled with 7CR-10. Scale bars = 20 μm for photos A-E (D and E taken at same magnification as C). Scale bar = 50 μm for photo F.
We were also unable to determine the cellular origin of these neural fibers. Two cells displayed faint labeling with 7CR-10, but this may have been nonspecific background signal, especially since no fibers were seen extending from these cells. Labeled fibers, however, passed through both nerve trunks (Figs. 1B and 2F). Elasmobranchs are particularly difficult for tracing neural connections and we have not yet succeeded in labeling cells in the brain or periphery with tracers placed in the main ganglion.
To control for cross-reactivity and to provide a tentative identification of the GnRH isoforms present, normal patterns of immunoreactivity with the two GnRH antisera were compared to those observed after preabsorption of the antisera with an excess of one of four isotypes of GnRH. Labeling with GF-5 antiserum (Fig. 3A-E) was clearly blocked by preabsorption with mammalian (mGnRH) GNRH-I or salmon (sGnRH) GnRH-III but not with lamprey GnRH (lGnRH). Preabsorption with chicken-II (cGnRH-II) (GnRH-II) decreased the intensity of GF-5 labeling, especially in the fiber bundles, but it did not completely block labeling. The percent cross-reactivities for the GF-5 antiserum reported by Lescheid et al. (1997) were 100% (mammalian), 68.8% (salmon), 3.9% (chicken-II), and <0.03% (lamprey). Labeling with 7CR-10 antiserum (Fig. 3F-J) was completely blocked with cGnRH-II) and sGnRH. There was strong 7CR-10 labeling (no apparent block) after preabsorption with mGnRH, but only a faint signal with lGnRH preabsorption. The percent cross-reactivities for the 7CR-10 antiserum reported by Lescheid et al. (1997) were 100% (chicken-II), 84.8% (salmon), 6.0% (lamprey), and <0.03% (mammalian).
The ChAT antiserum labeled distinct clusters of cells within the NT ganglion and only GF-5 labeling was specifically co-localized with ChAT positive cells in double label experiments (Fig. 2C). This co-localization was seen in every cell that was ChAT positive. Correspondingly there was no GF-5-ir cell that was not ChAT positive. Preabsorption with an excess of ChAT protein inhibited all labeling with ChAT antiserum (not shown). Neither FMRFa-ir cells (Fig. 2D) nor 7CR-10 labeled cell structures (Fig. 2E) were ChAT positive. Club-like structures labeled with 7CR-10 were often located among ChAT positive cells but were not labeled with ChAT antiserum. We attempted to double-label sections with the GF-5 and 7CR-10 antisera, to determine if their targets were co-localized. These antisera were both raised in rabbits so we used techniques for enzyme precipitation and antibody fragments that were designed to shield the first primary antiserum from further processing (Beltz and Burd, 1989). None of these techniques were successful in this tissue. Profiles resembling the club structures were not seen in GF-5 single-labeled tissue, although these might possibly have been missed among the many GF-5 labeled somata. More cogently, there were no profiles in double labeled tissue that were immunoreactive for GF-5 and not for ChAT.
3. DISCUSSION
This study found that two different GnRH isoforms are expressed in distinct neuronal processes in the nervus terminalis ganglion of bonnethead sharks. One isoform, which from its immunocytochemical profile is probably GnRH-II or dogfish (d)GNRH, is restricted to fibers and club-like anuclear profiles resembling enlarged nerve terminals, many of which abut blood vessels within the ganglion. The other isoform, probably mammalian (m)GNRH-I, is expressed in cells in the NT ganglion which also express choline acetyl-transferase (ChAT). Together with previously reported synaptic responses within the NT ganglion, these findings suggest possible functional interactions, involving GnRH, RF-amide peptides and acetyl choline, that may influence GnRH release.
It has been known for some time that the NT ganglion of bonnethead sharks contains two cell classes, delineated best by antibodies to either GnRH or FMRFamide/LPLRFamide-like (RF-amide) peptides (White and Meredith, 1995). Labeling with these two peptide markers has been described in the NT system of other elasmobranchs (Stell, 1984; Lovejoy et al., 1992a, 1992b; D’Antonio, et al. 1995; Chiba, 2000, Forlano et al 2000) as well as other vertebrate groups (Muske and Moore, 1988; Wirsig-Wiechmann and Basinger, 1988; Fisher et al., 1996; Wright and Demski, 1996). Less well known are the functional interactions of these two cell types, even though there are extensive synaptic connections within NT ganglia (White and Meredith, 1987; Zeng et al., 1990; Oka and Ichikawa, 1991). It seems possible that RF-amide peptides regulate GnRH release from the GnRH cells in NT as they do in the ventral forebrain of mammals and birds (Johnson and Frawley 2007; Simonneaux et al 2008, Clarke et al 2009). Although not conclusive of the relationship there is a striking similarity between the anatomy of RF-amide-ir fibers and GnRH-ir cells in bonnethead NT ganglion and mammalian forebrain (compare Fig 2b of White and Meredith 1995 and Fig 3 of Johnson and Frawley 2007) Little is known about other possible classes of cells influencing NT activity in elasmobranchs, although the two known cell-types seem to account for the vast majority of cells within the ganglion in bonnetheads (White and Meredith 1995).
The labeling with antiserum GF-5 illustrates the pattern and distribution of GnRH cells and fibers in the NT ganglion similar to those of other studies using different GnRH antisera (White and Meredith, 1995; Chiba, 2000). Principally, large clusters of labeled GnRH-ir cells were distributed throughout the ganglion, interspersed with other clusters of the RFa-ir cell type. Fibers of each these two immunoreactivities come into close contact with cells of the other type and form terminal or en-passant swellings that may include synaptic connections (White and Meredith 1995). On the basis of indirect but convergent information from anatomical and electrophysiological studies, we had earlier suggested that the RFa-ir cells were cholinergic and the GnRH-ir cells were not (White and Meredith 1995). The present results show clearly that the opposite is the case (see below).
The sparse labeling pattern revealed with the second GnRH antiserum, 7CR-10, was very surprising. The unusual labeling pattern suggested a possible functional division of GnRH cell types in the NT, which is supported by other immunocytochemical differences. Labeling of cell bodies within the ganglion by 7CR-10 antiserum was unconvincing. Strongly labeled varicose fibers, however, were observed scattered throughout each individual ganglion, as solitary fibers. The most unusual feature was the club-like structures. These structures were sometimes the size of cells but were clearly anuclear (Fig. 2A). Careful reconstruction of these structures from confocal-microscope optical sections suggested that they were nerve terminals. In shape they were similar to GnRH nerve terminals seen in the median eminence of rats (King and Rubin, 1995). Moreover, like those nerve terminals in rats, the GnRH-ir terminals in bonnethead NT ganglia were associated with blood vessels (Fig. 2B), suggesting a site of GnRH release into the general circulation. Further studies at the ultrastructure level will be needed to demonstrate that this association is consistent and has the appropriate microstructure for such a function.
The differences in GnRH-ir patterns are likely due to expression of two distinct GnRH isoforms. At least four isoforms of GnRH have been characterized in elasmobranchs (Lovejoy et al., 1992b; Calvin et al., 1993; D’Antonio et al., 1995). The preabsorption controls using commercially available GnRH peptides indicate that GF-5 antiserum may be labeling either sGnRH (GnRH-III), which the antibody was raised against, or mGnRH-I, with which it shows high cross-reactivity (Lescheid et al., 1997). Other antisera that were raised against mGnRH (a GnRH-I isoform) gave similar labeling patterns to that of GF-5 (White and Meredith, 1995). Furthermore, the cellular structures labeled by this antiserum and those labeled with 7CR-10 are separate. All the GF-5-ir cell structures and none of the 7CR-10-ir structures co-localized with ChAT antiserum labeling. Thus, they appear to be separate cellular components of the bonnethead NT and not a single cell type co-expressing two different GnRH isotypes. Since 7CR-10 cross-reacts well with sGnRH but does not label the GF-5-ir cells, the likely isoform in GF-5-ir cells is mGnRH (mammalian GnRH-I) and not sGnRH (GNRH-III). The data concerning the GnRH isoform labeled with 7CR-10 are less clear. This antiserum has a much narrower specificity (Lescheid et al., 1997; Forlano et al., 2000) and cross-reacted primarily with cGnRH-II (GnRH-II) and with dGnRH, which 7CR-10 was raised against. The dGnRH peptide was not available for this study and was not tested. Either one of these isoforms is a possible candidate for the GnRH in the club-like terminals but it does seem clear that it is not mGnRH. Forlano et al. (2000) have demonstrated a lamprey-like GnRH (lGnRH) immunoreactivity in a peripheral NT ganglion in the Atlantic Stingray. Here, the residual staining after preabsorption with lGnRH and the low cross reactivity of 7CR-10 with lGnRH (Lescheid et al 1997; Forlano et al., 2000) make it less likely that the structures stained with 7CR-10 in bonnethead shark contain a lamprey-like peptide. All the antibodies were purified as supplied, so artifacts due to cross reactivity with the conjugated peptides used to produce the antibodies are unlikely.
The two distinct GnRH isoforms and the anatomical difference of their distribution in the NT ganglion are presumably related to differences in function of these two cell types. It is now clear that most vertebrate groups express two or more isoforms of GnRH arising from two or three different embryonic origins (Schwanzel-Fukuda and Pfaff, 1989; Muske, 1993, Whitlock 2004 ), with different anatomical distributions in the brain (see introduction). The highly conserved chicken-GnRH-II isoform (GnRH-II) in the midbrain is widely distributed whereas other forms, including mGnRH (mGnRH-I), are more variable across taxa. The variable isoform, generally a GnRH-I or GnRH III isoform, distributed within the NT and preoptic systems, appears to be the one associated with gonadotropin release from the pituitary, where there is a pituitary portal system (Jennes and Stumpf, 1986; Lehman et al., 1987; Silverman et al, 1987; Forlano et al., 2000, see introduction). In elasmobranchs, where there is no pituitary portal system, there are at least four isoforms of GnRH that can be expressed but it is not clear which forms are playing an endocrine role or where they are released (Lovejoy et al, 1992a).
The 7CR-10-ir club-like structures in the NT ganglion may be a potential site for GnRH-II release into the general circulation. It is unknown where the cell bodies of these fibers are located, although several species of elasmobranchs express GnRH-II in a cluster of cells in the midbrain (Wright and Demski 1991; Forlano et al., 2000, see introduction). The GnRH-II isoform has an influence on reproductive physiology (Phillips et al., 1987; Millar, 2003) and behavior (Maney et al., 1997), possibly through synaptic release as a neuromodulator (Jones, 1987). GnRH-II-ir cells within the midbrain in elasmobranchs may influence the mechanics of mating by exerting some control over motor neurons of the male claspers (Demski, 1984) and these cells also have widespread connections with brainstem sensory nuclei (Forlano et al., 2000). GnRH-II (cGnRH-II) may play also an endocrine role (Miyamoto, 1984; Khakoo et al., 1994; Zohar et al, 1995). It does not appear to be the major form causing pituitary LH release in most vertebrates (Sharpe et al., 1990; Muske, 1993), but may help modulate relative LH and FSH release in mammals (Niell et al., 2001)
If, as in most species, the GnRH-I isoform (GF-5-ir) is the primary hypophysiotropic peptide, bonnethead sharks may possibly release two isoforms of GnRH into the general circulation: GnRH-I (mGnRH) somewhere in the ventral telencephalon and c GnRH-II (or dGnRH) from the NT ganglion. Control of reproductive development and behaviors may be enhanced by variations in the amount and timing in endocrine release of these two hormones, either by acting on the pituitary or directly on the reproductive organs (Millar, 2003). Numerous GnRH-I-ir fibers from the NT cells extend throughout the forebrain so the mGnRH-I, as well as GnRH-II, is likely acting also as a neuromodulator on neural systems within the brain (Oka and Matsushima, 1993; Moeller and Meredith, 1998). There are extensive synaptic connections within NT ganglia and neural activity in NT cells is influenced by efferent activity from the brain (White and Meredith 1987, 1993, 1995), so both GnRH release from NT GnRH cells and their neuromodulatory influence in the brain could be regulated both locally and centrally.
The two types of cell somata in bonnethead shark NT ganglia exhibit a difference in ChAT immunoreactivity. Clearly the large clusters of GnRH-ir cells labeled with GF-5 contain ChAT-ir and the clusters of LPLRFa-ir/FMRFa-ir (RFa-ir) cells do not. Thus GF-5-ir cells are likely to be cholinergic, as ChAT is one of the essential enzymes in the production of acetylcholine (ACh). In the dogfish also, there is widespread distribution of ChAT-ir, including cells in the retrobulbar region (Anadon et al., 2000) that may belong to the same population, and in rats, ChAT immunoreactivity is also localized in the nervus terminalis (Schwanzel-Fukuda et al 1986). In previous studies in the NT ganglia of bonnethead sharks both the GnRH-ir and RFa-ir somata displayed reactivity for acetylcholinesterase (AChE) (White and Meredith 1995). The RFa-ir cells were more strongly reactive and were previously (incorrectly) considered to be cholinergic. Other NT systems also show a distinction between cells that are strongly AChE-ir and those that are GnRH-ir (Wirsig and Leonard, 1986b; Caldani et al,, 1987). AChE markers are clearly useful in delineating cell populations in the NT, and may indicate important functional relationships. Markers for AChE and ChAT labeling often co-localize in various neural tissues and sometimes within individual cells (Mesulam, 1988; Illing, 1990; Criswell and Brandon, 1993). Both enzymes appear to be expressed by the prominent GnRH-ir cell population in bonnethead NT. There are also numerous examples where AChE is synthesized by non-cholinergic, non-ChAT-ir cells (Godrey et al, 1984; Levey et al., 1984; Pourcho and Osmank, 1986 including the RFa-ir cells in the NT. Such cells are generally considered to be cholinoceptive (LeJeune and Jourdan 1994, Mangoura et al 88) when associated with cholinergic cells. Thus, the RFa-ir cells could be responsive to ACh released by the GF5-ir GnRH cells.
Whether GnRH-ir cells synapse with RFa-ir cells or with each other to influence cell activity remains to be determined, but ACh inhibits one population of NT cells in vitro, possibly the RFa-ir cells, and excites another, possibly the GnRH cells (White and Meredith, 1995). These interactions may be important in regulating differences in endocrine release of the various isoforms of GnRH. In isolated GnRH producing cells from the hypothalamus, ACh will regulate the secretion of GnRH (Krsmanovic et al., 1998). In bonnethead sharks, the 7CR-10-ir club-like structures are usually observed in a cluster of GF-5-ir mGnRH-like cells, so there is the potential for direct interaction via ACh between the different GnRH-ir cell populations as well as between GF-5-ir cells and RFa-ir cells. If RF-amides are involved in regulating GnRH release in elasmobranchs as they are in mammals and birds (Johnson and Frawley 2007, Simonneaux et al 2008) the RFa-ir cells may influence GnRH release from processes immunoreactive for either GnRH isoform; and there is also the possibility of an ACh mediated feedforward effect on 7CR-10-ir processes or a feedback effect on GF-5-ir cells. Both RFa-ir cells and GnRH-ir cells also appear to be responsive to efferent control signals (White and Meredith 1987, 93, 95), so GnRH functions may be influenced by both intra and extra-ganglionic circuits. GF-5-ir cells may regulate GnRH secretion from the NT by influencing activity or peptide release of 7CR-10-ir cells directly via ACh, or indirectly via RFa; or they may affect GnRH secretion from their own cell type through an autocrine-like effect or, via a feedback loop, from RFa cells. Further studies will be needed to flesh out these potential interactions, both in their synaptic connections and potential hormonal activities.
4. EXPERIMENTAL PROCEDURE
Adult bonnethead sharks, Sphyrna tiburo, were captured with a 100m trammel net along the Northern Gulf coast of Florida, near shore in 2m depth. Live specimens were quickly brought back to the Florida State University Marine Laboratory and maintained in a 6m annular tank. The FSU Animal Care and Use Committee, in accordance with NIH guidelines, approved all animal treatments.
For immuno-cytochemistry studies, sharks were anesthetized with tricaine methanesulfonate (MS222 at 1:1000), sexed, and weighed. The animals were perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) after injection with heparin. The nervus terminalis, along with the brain and olfactory tract, were dissected out and placed into the same fixative overnight. The ganglia of the NT were later placed into gelatin and sectioned with a vibratome at 25–35 um.
All immunocytochemical procedures involved a standard two-step process with fluorescent markers (Beltz and Burd, 1989). Primary antibodies included GF-5 (1:5000), raised in rabbit against salmon GnRH, and 7CR-10 (1:3000), raised in rabbit against dogfish GnRH (gifts from Dr. Nancy Sherwood). Antibodies to FMRFamide (DiaSorin, Inc.) where also raised in rabbit and used at a 1:100 dilution. Cholinergic cells were labeled with an antiserum (144P; 1:100) raised in goat against ChAT (Chemicon, Inc.). Some sections were double labeled with 7CR-10 and Hoechst dye (for DNA) at 10 μg/ml (Molecular Probes, Inc.). All antibodies were purified, as supplied. All sections were first presoaked in PBS containing 3% normal donkey serum and 0.4% Triton X-100. All subsequent steps involved solutions in 0.1 M PBS at room temperature. Sections were incubated in primary antiserum for 24 hours at room temperature, washed, and then incubated for an additional 18–24 hours with donkey anti-rabbit or donkey anti-goat secondary antibody conjugated to either Cy3 or FITC. For double-labeled procedures the two primary antisera were incubated sequentially. Washed sections were mounted on slides and allowed to dry overnight. Slides were cover slipped with vectashield (Vector Laboratories, Inc.) and sealed with nail polish for later examination with a Zeiss LSM 410 scanning confocal microscope. All images were digitally collected with Zeiss software and later printed using Adobe Photoshop and a Kodak dye sublimation printer. Some minor manipulations of contrast and brightness were employed during this final stage but all such manipulations were applied uniformly to the entire micrograph.
As a control for nonspecific binding, incubation with each primary antiserum reported here was omitted during the processing of some tissue sections. No fluorescent signals could be detected in any of these sections. To test for antiserum specificity, some sections were processed with primary antiserum that was first incubated for 24 hours with the target protein (10–100× the molar concentration of the primary antisera). For both GnRH antisera this involved incubation with several isoforms of GnRH (Sigma): mammalian (m = mGnRH-I), chicken-II (c = GnRH-II), salmon (s = GnRH-III), and lamprey (l). Sections were then processed as above with these preabsorbed antisera and with normal antisera, in parallel. During documentation of GnRH preabsorption-controls (Fig. 3), settings on the confocal microscope (e.g. brightness and contrast) were adjusted appropriately to show GnRH-ir for sections that were processed normally (no preabsorption). These settings were then maintained when documenting all preabsorption sections of a given primary antiserum, to minimize biases during capture and printing of digital images.
Acknowledgments
We thank Nancy Sherwood for the generous gifts of GnRH antisera. We also thank Anna Lerant for her invaluable advice with ICC procedures, Kim Riddle for her assistance with the confocal microscope and Jody Myers for both technical and intellectual contributions. This study was conducted at the Florida State University Coastal and Marine Laboratory and was supported by NIDCD grants DC000471, and DC000906, and manuscript preparation by DC005813.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE REFERENCES
- Anadon R, Molist P, Rodriguez-Moldes I, Lopez JM, Quintela I, Cervino MC, Barja P, Gonzalez A. Distribution of choline acetyltransferase immunoreactivity in the brain of an elasmobranch, the lesser spotted dogfish (Scylliorhinus canicula) J Comp Neurol. 2000;420:139–70. doi: 10.1002/(sici)1096-9861(20000501)420:2<139::aid-cne1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Beltz BS, Burd GD. Immunocytochemistry techniques: principles and practice. Cambridge, MA: Blackwell Scientific; 1989. [Google Scholar]
- Blake CB, Meredith M. Selective enhancement of main olfactory input to the medial amygdala by GnRH. Brain Res. 2010;1317:46–59. doi: 10.1016/j.brainres.2009.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldani M, Batailler M, Jordan F. The sheep terminal nerve: coexistence of LHRH- and AChE-containing neurons. Neurosci Lett. 1987;83:221–226. doi: 10.1016/0304-3940(87)90089-9. [DOI] [PubMed] [Google Scholar]
- Calvin JL, Slater CH, Bolduc TG, Laudano AP, Sower SA. Multiple molecular forms of gonadotropin-releasing hormone in the brain of an elasmobranch: evidence for IR-lamprey GnRH. Peptides. 1993;14:725–729. doi: 10.1016/0196-9781(93)90104-o. [DOI] [PubMed] [Google Scholar]
- Chiba A. Immunohistochemical cell types in the terminal nerve ganglion of the cloudy dogfish, Scylliorhinus torazame, with special regard to neuropeptide Y/FMRFamide-immunoreactive cells. Neurosci Lett. 2000;286:195–198. doi: 10.1016/s0304-3940(00)01122-8. [DOI] [PubMed] [Google Scholar]
- Chriswell MH, Brandon C. Acetylcholinesterase and choline acetyltransferase localization patterns do correspond in cat and rat retinas. Vision Res. 1993;33:1747–1753. doi: 10.1016/0042-6989(93)90165-s. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Qi I, Sari IP, Smith JT. Evidence that RF-amide related peptides are inhibitors of reproduction in mammals. Frontiers in Neuroendocrinology. 2009;30:371–378. doi: 10.1016/j.yfrne.2009.04.001. [DOI] [PubMed] [Google Scholar]
- D’Antonio M, Vallarino M, Lovejoy DA, Vandesande F, King JA, Pierantoni R, Peter RE. Nature and distribution of gonadotropin-releasing hormone (GnRH) in the brain, and GnRH and GnRH binding activity in serum of the spotted dogfish Scyliorhinus canicula. Gen Comp Endocrinol. 1995;98:35–49. doi: 10.1006/gcen.1995.1042. [DOI] [PubMed] [Google Scholar]
- Demski LS. The evolution of neurological substrates of reproductive behavior: sex-steroid and LHRH-specific pathways including the terminal nerve. Am Zool. 1984;24:809–830. [Google Scholar]
- Demski LS, Fields RD, Bullock TH, Schreibman MP, Margolis-Nunno H. The terminal nerve of sharks and rays: electron microscopic, immunocytochemical and electrophyiological studies. In: Demski LS, Schwanzel-Fukuda M, editors. The terminal nerve (nervus terminalis): structure, function, and evolution. New York: Ann NY Acad Sci; 1987. pp. 323–333. [Google Scholar]
- Demski LS, Northcutt RG. The terminal nerve: A new chemosensory nerve in vertebrates? Science. 1983;220:435–437. doi: 10.1126/science.6836287. [DOI] [PubMed] [Google Scholar]
- Dodd JM. Reproduction in cartilaginous fishes (Chondricthyes) In: Hoar WS, Randall DJ, Donaldson EM, editors. Fish Physiology Vol IX-A. New York: Academic Press; 1983. pp. 31–95. [Google Scholar]
- Dorsa DM, Smith ER. Facilitation of mounting behavior in male rats by intracranial injections of luteinizing hormone-releasing hormone. Regul Pept. 1980;1 (2):147–155. doi: 10.1016/0167-0115(80)90017-8. [DOI] [PubMed] [Google Scholar]
- Dubois EA, Zandbergen MA, Peute J, Goos HJ. Evolutionary development of three gonadotropin-releasing hormone (GnRH) systems in vertebrates. Brain Res Bull. 2002;57:413–418. doi: 10.1016/s0361-9230(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Fernald RD, White RB. Gonadotropin-Releasing Hormone Genes: Phylogeny, Structure, and Functions. Frontiers in Neuroendocrinology. 1999;20:224–240. doi: 10.1006/frne.1999.0181. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fewell GD, Meredith M. Facilitation of mating behavior in male hamsters by LHRH and AcLHRH5–10: interaction with the vomeronasal system. Physiol Behav. 1995;57:213–221. doi: 10.1016/0031-9384(94)00276-b. [DOI] [PubMed] [Google Scholar]
- Fisher AJ, Reisch HM, Kyle AL, Stell WK. Characterization of the Rfamide-like neuropeptides in the nervus terminalis of the goldfish(Carassius auratus) Regul Peptide. 1996;62:73–87. doi: 10.1016/0167-0115(95)00165-4. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Maruska KP, Sower SA, King JA, Tricas TC. Differential distribution of gonadotropin-releasing hormone-immunoreactive neurons in the stingray brain: functional and evolutionary considerations. Gen Comp Endocrinol. 2000;118:226–48. doi: 10.1006/gcen.2000.7467. [DOI] [PubMed] [Google Scholar]
- Godfrey DA, Park JL, Ross CD. Choline acetyltransferase and acetylcholinesterase in centrifugal labyrinthine bundles of rats. Hear Res. 1984;14:93–106. doi: 10.1016/0378-5955(84)90072-8. [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Sower SA, Ronsheim PM, Connolly JB, Bourn CG, Rubin BS. Guinea Pig GnRH: Localization and Physiological Activity Reveal That It, Not Mammalian GnRH, Is the Major Neuroendocrine Form in Guinea Pigs. Endocrinology. 2002;143:1602–1612. doi: 10.1210/endo.143.5.8803. [DOI] [PubMed] [Google Scholar]
- Illing RB. Choline acetyltransferase-like immunoreactivity in the superior colliculus of the cat and its relation to the pattern of acetylcholinesterase staining. J Comp Neurol. 1990;296:32–46. doi: 10.1002/cne.902960104. [DOI] [PubMed] [Google Scholar]
- Jennes L, Stumpf WE. Gonadotropin-releasing hormone immunoreactive neurons with access to fenestrated capillaries in mouse brain. Neuroscience. 1986;18:403–416. doi: 10.1016/0306-4522(86)90162-4. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Hormones and Behavior. 2007;51:171–180. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SW. Chicken II luteinizing hormone-releasing hormone inhibits the M-current of bullfrog sympathetic neurons. Neurosci Lett. 1987;80:180–184. doi: 10.1016/0304-3940(87)90650-1. [DOI] [PubMed] [Google Scholar]
- Khakoo Z, Bhatia A, Gedamu L, Habibi HR. Functional specificity for salmon gonadotropin-releasing hormone (GnRH) and chicken GnRH-II coupled to the gonadotropin release and subunit messenger ribonucleic acid level in the goldfish pituitary. Endocrinology. 1994;134:838–847. doi: 10.1210/endo.134.2.7507838. [DOI] [PubMed] [Google Scholar]
- Kim KH, Patel L, Tobet SA, King JC, Rubin BS, Stopa EG. Gonadotropin-releasing hormone immunoreactivity in the adult and fetal human olfactory system. Brain Res. 1999;826:220–229. doi: 10.1016/s0006-8993(99)01271-8. [DOI] [PubMed] [Google Scholar]
- King JC, Miller RP. Evolutionary aspects of gonadotropin-releasing hormone and its receptor. Cell Mol Neurobiol. 1995;15:5–23. doi: 10.1007/BF02069556. [DOI] [PubMed] [Google Scholar]
- King JC, Rubin BS. Dynamic alterations in luteinizing hormone-releasing homone (LHRH) neuronal cell bodies and terminals of adult rats. Cell Mol Neurobiol. 1995;15:89–106. doi: 10.1007/BF02069560. [DOI] [PubMed] [Google Scholar]
- Koza JM, Wirsig-Wiechmann CR. A subpopulation of nervus terminalis neurons projects to the olfactory mucosa in Xenopus laevis. J Neurosci Res. 2001;66:8–15. doi: 10.1002/jnr.1192. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AA, Inoue K, Ukena K, Tsutsui K, Silver R. PNAS. 2006;103:2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krsmanovic LZ, Mores N, Navarro CE, Saeed SA, Arora KK, Catt KJ. Muscarinic regulation of intracellular signaling and neurosecretion in gonadotropin-releasing hormone neurons. Endocrinology. 1998;139:4037–4043. doi: 10.1210/endo.139.10.6267. [DOI] [PubMed] [Google Scholar]
- Latimer VS, Rodrigues SM, Garyfallou VT, Kohama SG, White RB, Fernald RD, Urbanski HF. Two molecular forms of gonatotropin-releasing hormone (GnRH-I and GnRH-II) are expressed by two separate populations of cells in the rhesus monkey hypothalamus. Brain Res Mol Brain Res. 2000;75:287–292. doi: 10.1016/s0169-328x(99)00316-2. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Newman SW, Silverman AJ. Luteinizing hormone-releasing hormone in the vomeronasal system and terminal nerve of the hamster. Ann NY Acad Sci. 1987;519:229–240. doi: 10.1111/j.1749-6632.1987.tb36300.x. [DOI] [PubMed] [Google Scholar]
- LeJeune H, Jourdan F. Acetylcholinesterase-containing intrinsic neurons in the rat main olfactory bulb: cytological and neurochemical features. Eur J Neurosci. 1994;6:1432–44. doi: 10.1111/j.1460-9568.1994.tb01005.x. [DOI] [PubMed] [Google Scholar]
- Lescheid DW, Terasawa E, Abler LA, Urbanski HF, Warby CM, Millar RP, Sherwood NM. A second form of gonadotropin-releasing hormone (GnRH) with characteristics of chicken GnRH-II is present in the primate brain. Endocrinology. 1997;138:5618–5629. doi: 10.1210/endo.138.12.5592. [DOI] [PubMed] [Google Scholar]
- Levey AI, Wainer BH, Rye DB, Mufson EJ, Mesulam MM. Choline acetyltransferase-immunoreactive neurons intrinsic to rodent cortex and distinction from acetylcholinesterase-positive neurons. Neuroscience. 1984;13:341–353. doi: 10.1016/0306-4522(84)90234-3. [DOI] [PubMed] [Google Scholar]
- Lovejoy DA, Ashmead BJ, Coe IR, Sherwood NM. Presence of gonadotropin-releasing hormone immunoreactivity in dogfish and skate brains. J Exp Biol. 1992a;263:272–283. [Google Scholar]
- Lovejoy DA, Stell WK, Sherwood NM. Partial characterization of four forms of immunoreactive gonadotropin-releasing hormone in the brain and terminal nerve of the spiny dogfish (Elasmobranchii; Squalus acanthias) Regul Peptides. 1992b;37:39–48. doi: 10.1016/0167-0115(92)90062-y. [DOI] [PubMed] [Google Scholar]
- Maney DL, Richardson RD, Wingfield JC. Central administration of chicken gonadotropin-releasing hormone-II enhances courtship behavior in a female sparrow. Horm Behav. 1997;32:11–18. doi: 10.1006/hbeh.1997.1399. [DOI] [PubMed] [Google Scholar]
- Mangoura D, Sakellaridis N, Vernadakis A. Cholinergic neurons in cultures derived from three-, six- or eight-day-old chick embryo: a biochemical and immunocytochemical study. Brain Res. 1988;468:37–46. doi: 10.1016/0165-3806(88)90005-3. [DOI] [PubMed] [Google Scholar]
- Meredith M, White J. Interactions between the olfactory system and the terminal nerve. In: Demski LS, Schwanzel-Fukuda M, editors. The terminal nerve (nervus terminalis): structure, function, and evolution. New York: Ann NY Acad Sci; 1987. pp. 349–368. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J Comp Neurol. 1988;275:216–240. doi: 10.1002/cne.902750205. [DOI] [PubMed] [Google Scholar]
- Millar RP. GnRH-II and GnRH-II receptors. Trends in Endocrinol Metab. 2003;14:36–43. doi: 10.1016/s1043-2760(02)00016-4. [DOI] [PubMed] [Google Scholar]
- Millar RP, Lu Z-L, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-Releasing Hormone Receptors. Endocrine Reviews. 2004;25:235–275. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Hasegawa Y, Nomura M, Igarashi M, Kangawa K, Matsuo H. Identification of the second gonadotropin-releasing hormone in chicken hypothalamus: evidence that gonadotropin secretion is probably controlled by two distinct gonadotropin-releasing hormones in avian species. Proc Natl Acad Sci USA. 1984;81:3874–3878. doi: 10.1073/pnas.81.12.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller JF, Meredith M. Increase in gonadotropin-releasing hormone (GnRH) levels in CSF after stimulation of the nervus terminalis in Atlantic stingray, Dasyatis sabina. Brain Res. 1998;806:104–107. doi: 10.1016/s0006-8993(98)00683-0. [DOI] [PubMed] [Google Scholar]
- Moeller JF, Myers JM, Meredith M. Distribution of LHRH isotypes in nervus terminalis ganglion of bonnethead shark and Atlantic stingray. Neurosci Abstr. 1997;23:143. [Google Scholar]
- Moss RL, Dudley CA. Neural Control of Reproductive Function. John Wiley & Sons; 1989. Luteinizing hormone-releasing hormone (LHRH): peptidergic signals in the neural integration of female reproductive behavior; pp. 485–499. [Google Scholar]
- Munz H, Claas B, Stumpf WE, Jennes L. Centrifugal innervation of the retina by luteinizing hormone releasing hormone (LHRH)-immunoreactive telencephalic neurons in teleostean fishes. Cell Tissue Res. 1982;222:313–323. doi: 10.1007/BF00213215. [DOI] [PubMed] [Google Scholar]
- Muske LE. Evolution of gonadotropin-releasing hormone (GnRH) neuronal systems. Brain Behav Evol. 1993;42:215–230. doi: 10.1159/000114156. [DOI] [PubMed] [Google Scholar]
- Muske LE, Moore FL. The nervus terminalis in amphibians: Anatomy, chemistry and relationship with the hypothalamic gonadotropin- releasing hormone system. Brain Behav Evol. 1988;32:141–150. doi: 10.1159/000116541. [DOI] [PubMed] [Google Scholar]
- Muske LE, King JA, Moore FL, Millar RP. Goanadotropin-releasing hormones in microdissected brain regions of an amphibian: concentration and anatomical distribution of immunoreactive mammalian GnRH and chicken GnRH II. Regul Peptides. 1994;54:373–384. doi: 10.1016/0167-0115(94)90535-5. [DOI] [PubMed] [Google Scholar]
- Niell JD, Duck LW, Sellars JC, Musgrove LC. A gonadotropin-releasing hormone (GnRH) receptor specific for GnRH-II in primates. Biochem Biophys Res Comm. 2001;282:1012–1018. doi: 10.1006/bbrc.2001.4678. [DOI] [PubMed] [Google Scholar]
- Nozaki M, Tsukahara T, Kobayashi H. An immunocytochemical study on the distribution of neuropeptides in the brain of certain species of fish. Biomed Res Suppl. 1984;4:135–145. [Google Scholar]
- Nozaki M. Tissue distribution of hormonal peptides in primitive fishes. In: Foreman RE, Gorbman A, Dodd JM, Olsson R, editors. Evolutionary Biology of Primitive Fishes. New York: Plenum Press; 1985. pp. 433–454. [Google Scholar]
- Oka Y, Ichikawa M. Ultrastructure of the ganglion cells of the terminal nerve in the dwarf gourami (Coliosa lalai) J Comp Neurol. 1991;304:161–171. doi: 10.1002/cne.903040202. [DOI] [PubMed] [Google Scholar]
- Oka T, Matsushima T. Gonadotropin-releasing hormone (GnRH)-immunoreactive terminal nerve cells have intrinsic rhythmicity and project widely in the brain. J Neurosci. 1993;13:2161–2176. doi: 10.1523/JNEUROSCI.13-05-02161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y. Three Types of Gonadotrophin-Releasing Hormone Neurones and Steroid-Sensitive Sexually Dimorphic Kisspeptin Neurones in Teleosts. J Neuroendocrinol. 2009;21:334–338. doi: 10.1111/j.1365-2826.2009.01850.x. [DOI] [PubMed] [Google Scholar]
- Palevitch O, Abraham E, Borodovsky N, Levkowitz G, Zohar Y, Gothilf Y. Nasal Embryonic LHRH Factor Plays a Role in the Developmental Migration and Projection of Gonadotropin-Releasing Hormone 3 Neurons in Zebrafish. Developmental Dynamics. 2009;238:66–75. doi: 10.1002/dvdy.21823. [DOI] [PubMed] [Google Scholar]
- Pan JT, Kow LM, Pfaff DW. Modulatory actions of Luteinizing Hormone Releasing Hormone on electrical activity of preoptic neurons in brain slices. Neuroscience. 1988;27:623–628. doi: 10.1016/0306-4522(88)90293-x. [DOI] [PubMed] [Google Scholar]
- Pandolfi M, Muñoz-Cueto JA, Lo-Nostro FL, Downs JL, Paz DA, Maggese MC, Urbanski HF. GnRH systems of Cichlasoma dimerus (Perciformes, Cichlidae) revisited: a localization study with antibodies and riboprobes to GnRH-associated peptides. Cell Tissue Res. 2005;321:219–232. doi: 10.1007/s00441-004-1055-7. [DOI] [PubMed] [Google Scholar]
- Park D, Eisthen HL. Gonadotropin-releasing hormone (GnRH) modulates odorant responses in peripheral olfactory system of axolotls. J Neurophysiol. 2003;90:731–738. doi: 10.1152/jn.01162.2002. [DOI] [PubMed] [Google Scholar]
- Peter RE, Habibi HR, Marchant TA, Nahornick CS. Vertebrate gonadotropin-releasing hormones: phylogeny and structure-function relationships. In: Demski LS, Schwanzel-Fukuda M, editors. The terminal nerve (nervus terminalis): structure, function, and evolution. New York: Ann NY Acad Sci; 1987. pp. 299–309. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Jorgenson K, Kow L-M. Luteinizing hormone-releasing hormone in rat brain: Gene expression, role as neuromodulator, and functional effects. In: Demski LS, Schwanzel-Fukuda M, editors. The terminal nerve (nervus terminalis): structure, function, and evolution. New York: Ann NY Acad Sci; 1987. pp. 323–333. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hostetter G, Kerdelhue B, Kozlowski GP. Immunocytochemical localization of LHRH in central olfactory pathways of hamster. Brain Res. 1980;193:574–579. doi: 10.1016/0006-8993(80)90192-4. [DOI] [PubMed] [Google Scholar]
- Pourcho RG, Osman K. Acetylcholinesterase localization in cat retina: a comparison with choline acetyltransferase. Exp Eye Res. 1986;43:585–594. doi: 10.1016/s0014-4835(86)80025-2. [DOI] [PubMed] [Google Scholar]
- Phillips JA, Frye F, Bercovitz A, Calle P, Millar R, Rivier J, Lasley BL. Exogenous GnRH overrides the endogenous annual reproductive rhythm in green iguana, Iguana iguana. J Exp Zool. 1987;241:227–236. doi: 10.1002/jez.1402410209. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Alones VE, Craig-Veit CB, Millam JR. Distribution of chicken-II gonadotropin-releasing hormone in mammalian brain. J Comp Neurol. 1995;357:524–531. doi: 10.1002/cne.903570404. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Silverman AJ. The nervus terminalis of the guinea pig: A new luteinizing hormone-releasing hormone (LHRH) neuronal system. J Comp Neurol. 1980;191:213–225. doi: 10.1002/cne.901910205. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing-releasing hormone neurons. Nature. 1989;338:161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Morell JI, Pfaff DW. Localization of choline acetyltransferase and vasoactive intestinal polypeptide-like immunoreactivity in the nervus terminalis of the fetal and neonatal rat. Peptides. 1986;7:899–906. doi: 10.1016/0196-9781(86)90112-9. [DOI] [PubMed] [Google Scholar]
- Sharpe PJ, Talbot RT, Main GM, Dunn IC, Fraser HM, Huskisson NS. Physiological roles of chicken LHRH-I and –II in the control of gonadotropin release in the domestic chicken. J Endocrinol. 1990;124:291–299. doi: 10.1677/joe.0.1240291. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Lovejoy DA, Coe IR. Origin of mammalian gonadotropin-releasing hormones. Endocrine Rev. 1993;14:241–254. doi: 10.1210/edrv-14-2-241. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Jhamandas J, Renaud LP. Localization of luteinizing hormone-releasing hormone (LHRH) neurons that project to the median eminence. J Neurosci. 1987;7:2312–2319. [PMC free article] [PubMed] [Google Scholar]
- Simonneaux V, Ansel L, Revel FG, Klosen P, Pe′vet P, Mikkelsen JD. Kisspeptin and the seasonal control of reproduction in hamsters. Peptides. 2008 doi: 10.1016/j.peptides.2008.06.006. in press. [DOI] [PubMed] [Google Scholar]
- Stell WK. Luteinizing hormone-releasing hormone (LHRH)- and pancrceatic polypeptide (PP)-immunoreactive neurons in the terminal nerve of spiny dogfish, Squalus acanthias. Anat Rec. 1984;208:173A–174A. [Google Scholar]
- Temple JL, Millar RP, Rissman EF. An evolutionarily conserved form of gonadotropin-releasing hormone coordinates energy and reproductive behavior. Endocrinol. 2003;144:13–19. doi: 10.1210/en.2002-220883. [DOI] [PubMed] [Google Scholar]
- Troskie B, Illing N, Rumbak E, Sun YM, Hapgood J, Sealfon S, Conklin D, Millar R. Identification of three putative GnRH receptor subtypes in Vertebrates. Gen Comp Endocrinology. 1998;112:296–302. doi: 10.1006/gcen.1998.7156. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Bentley GE, Kriegesfeld LJ, Osugi T, Seong JY, Vaudrey H. Discovery and evolutionary history of gonadotropin-inhibitory hormone and kisspeptin: new key neuropeptides controlling reproduction. J Neuroendocrinology. 2010;22:716–727. doi: 10.1111/j.1365-2826.2010.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Meredith M. Synaptic interactions in the nervus terminalis ganglion of elasmobranchs. In: Demski LS, Schwanzel-Fukuda M, editors. The terminal nerve (nervus terminalis): structure, function, and evolution. New York: Ann NY Acad Sci; 1987. pp. 33–49. [DOI] [PubMed] [Google Scholar]
- White J, Meredith M. Spectral analysis and modeling of ACh and NE effects on shark nervus terminalis activity. Brain Res Bull. 1993;31:369–374. doi: 10.1016/0361-9230(93)90229-5. [DOI] [PubMed] [Google Scholar]
- White J, Meredith M. Nervus terminalis ganglion of the bonnethead shark (Sphyrna tiburo): evidence for cholinergic and catecholaminergic influence on two cell types distinguished by peptide immunocytochemistry. J Comp Neurol. 1995;351:385–403. doi: 10.1002/cne.903510306. [DOI] [PubMed] [Google Scholar]
- Whitlock KE. Development of the Nervus Terminalis: Origin and Migration. Micros Res And Technique. 2004;65:2–12. doi: 10.1002/jemt.20094. [DOI] [PubMed] [Google Scholar]
- Wirsig-Wiechmann CR. Nervus terminalis lesions: I. No effect on pheromonally induced testosterone surges in the male hamster. Physiol Behav. 1993;53:251–255. doi: 10.1016/0031-9384(93)90201-p. [DOI] [PubMed] [Google Scholar]
- Wirsig-Wiechmann CR. Nervus terminalis lesions: II. Enhancement of lordosis induced by tactile stimulation in the hamster. Physiol Behav. 1997;61:867–871. doi: 10.1016/s0031-9384(96)00610-5. [DOI] [PubMed] [Google Scholar]
- Wirsig-Wiechmann CR, Basinger SF. FMRFamide-immunoreactive retinopetal fibers in the frog, Rana pipiens: Demonstration by lesion and immunocytochemical techniques. Brain Res. 1988;449:116–134. doi: 10.1016/0006-8993(88)91030-x. [DOI] [PubMed] [Google Scholar]
- Wirsig CR, Getchell TV. Amphibian terminal nerve: Distribution revealed by LHRH and AchE markers. Brain Res. 1986;385:10–21. doi: 10.1016/0006-8993(86)91541-6. [DOI] [PubMed] [Google Scholar]
- Wirsig CR, Leonard CM. The terminal nerve projects centrally in the hamster. Neuroscience. 1986a;19:709–717. doi: 10.1016/0306-4522(86)90294-0. [DOI] [PubMed] [Google Scholar]
- Wirsig CR, Leonard CM. Acetylcholinesterase and luteinizing hormone-releasing hormone distinguish separate populations of terminal nerve neurons. Neuroscience. 1986b;19:719–740. doi: 10.1016/0306-4522(86)90295-2. [DOI] [PubMed] [Google Scholar]
- Wirsig CR, Leonard CM. Terminal nerve damage impairs the mating behavior of the male hamster. Brain Res. 1987;417:293–303. doi: 10.1016/0006-8993(87)90454-9. [DOI] [PubMed] [Google Scholar]
- Wright DE, Demski LS. Gonadotropin hormone-releasing hormone (GnRH) immunoreactivity in the mesencephalon of sharks and rays. J Comp Neurol. 1991;307:49–56. doi: 10.1002/cne.903070105. [DOI] [PubMed] [Google Scholar]
- Wright DE, Demski LS. Organization of GnRH and FMRF-amide systems in two primitive bony fishes (Order Polypteriformes) Brain Behav Evol. 1996;47:267–278. doi: 10.1159/000113246. [DOI] [PubMed] [Google Scholar]
- Zeng L-M, Pfaff DW, Schwanzel-Fukuda M. Synaptology of luteinizing hormone-releasing hormone (LHRH)-immunoreactive cells in the nervus terminalis of the gray short-tailed opossum (Monodelphis domestica) J Comp Neurol. 1990;295:327–337. doi: 10.1002/cne.902950213. [DOI] [PubMed] [Google Scholar]
- Zhang W, Delay RJ. Gonadotropin-Releasing Hormone Modulates Voltage-Activated Sodium Current and Odor Responses in Necturus maculosus Olfactory Sensory Neurons. J Neurosci Res. 2007;85:1656–1667. doi: 10.1002/jnr.21297. [DOI] [PubMed] [Google Scholar]
- Zohar Y, Elizur A, Sherwood NM, Powell JFF, Rivier JE, Zmora N. Gonadotropin-releasing activities of the three native forms of gonadotropin-releasing hormones present in the brain of gilthead seabream, Sparus aurata. Gen Comp Endocrinol. 1995;97:289–299. doi: 10.1006/gcen.1995.1029. [DOI] [PubMed] [Google Scholar]


