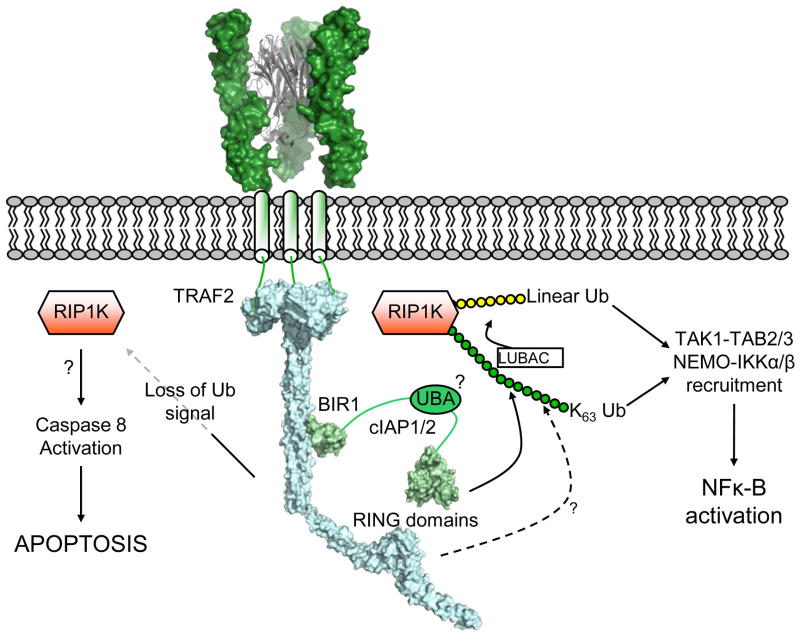
Figure 4. Signaling at the cytoplasmic face of the TNF receptor.
Illustrative representation of the complex signaling network assembled upon receptor ligation by TNF ligands. Trimerization of the TNF receptor (based on [80]) recruits TRAF2 to the cytoplasmic tail of the receptor. cIAP1 and cIAP2 are subsequently recruited through the coiled-coil domain of TRAF2, and as E3 ligases cIAP1/2 (and possibly TRAF2) facilitate ubiquitination of various substrates using their RING domains. K63-linked ubiquitin chains on RIP1K serve as a platform for recruitment of proteins containing ubiquitin-binding domains such as NEMO, TAB2 and IAP proteins themselves. Loss of this ubiquitin signal by degradation of cIAPs or other means leaves RIP1K free to activate Caspase-8, in a manner that is currently not clear at a structural level. (Based upon PDB entries: 1tnr, 1ca9, 3m0a, 3hcs, 3eb6).