Abstract
Investigation of the endemic Malagasy plant Bussea sakalava Du Puy & R. Rabev. (Fabaceae) for antiproliferative activity against the A2780 ovarian cancer cell line led to the isolation of the four new diphenylpropanes 1–4 and the new cycloheptadibenzofuran 5; compound 5 has a previously unreported natural product skeleton. The structure elucidation of these compounds was based on the analysis of their 1D and 2D NMR and mass spectroscopic data. Compounds 1–5 were tested for antiproliferative activity against the A2780 human ovarian cancer cell line.
In our continuing search for biologically active natural products from tropical rainforests as part of an International Cooperative Biodiversity Group (ICBG) program, we obtained an ethanol extract from the roots of a plant identified as Bussea sakalava Du Puy & R. Rabev. (Fabaceae) from Madagascar. This extract showed moderate antiproliferative activity against the A2780 human ovarian cancer cell line with an IC50 value of 10 µg/mL. The extract was selected for examination on the basis of this activity and the absence of previous phytochemical studies of the species.
Previous studies on the genus Bussea indicated the presence of azetidine-2-carboxylic acid and 3-hydroxyproline in seeds of different Bussea species,2,3 and the cytotoxicity and high trypanocidal activity of a methanol extract of stem bark of Bussea occidentalis has been reported.4
Fractionation of a dichloromethane fraction of an ethanol extract of B. sakalava by C-18 open column and high performance liquid chromatography (HPLC) yielded four new diphenylpropanes named bussealins A–D (1 – 4) and a cycloheptadibenzofuran derivative named bussealin E (5). Herein we report the structural elucidation of these new compounds and their antiproliferative properties against the A2780 human ovarian cancer cell line.
Results and Discussion
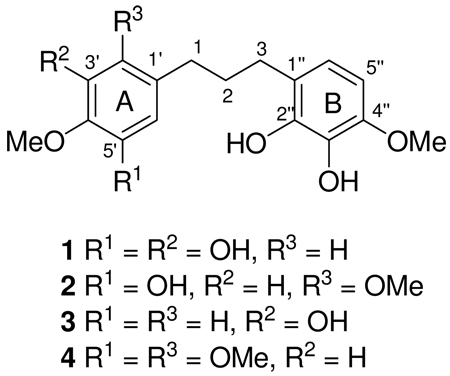
Bussealin A (1) was obtained as an off-white amorphous solid. Its positive ESI-MS revealed a pseudomolecular ion peak at m/z 321.1338 [M + H]+ corresponding to the molecular formula C17H21O6. The IR spectrum showed absorptions of OH (3367 cm−1) and aromatic groups. The 1H NMR spectrum (Table 1) exhibited a singlet at δH 6.18 (2H, s) corresponding to a pair of aromatic protons of an A2 system, two aromatic doublets [δH 6.50 (d, J = 8.4) an 6.38 (d, J = 8.4)] of an AB system, two OCH3 groups [δH 3.75 (s) and 3.78 (s)], and a multiplet and two triplet methylene groups at δH 1.79 (2H, m), 2.52 (2H, t, J = 7.7) and 2.41 (2H, t, J = 7.7) respectively. The 13C NMR spectrum of 1 exhibited signals for 17 carbons, including three methylene carbons (δC 36.5, 33.0, and 30.6), two OCH3 groups (δC 56.5 and 60.8), and twelve aromatic carbons assignable to two isolated aromatic rings. Six of the aromatic carbons were oxygenated, as shown by their deshielded carbon chemical shifts (Table 1) and were consistent with the molecular formula. The above data suggested that 1 had a diphenyl propane skeleton. The complete 1H and 13C NMR assignments and the connectivities were determined from analysis of a combination of COSY, HMQC, and HMBC data. Three mutually coupled methylene groups were revealed by the cross peaks observed in the COSY spectrum. In the HMBC spectrum, H-1 (δH 2.41) showed correlations with C-2 (δC 33.0), C-3 (δC 30.6), C-1' (δC 140.2), and with C-2' and C-6', both of which had the same chemical shifts (δC 108.7). The A2 substitution pattern of the A ring of 1 was established by HMBC correlations from the signal at δH 6.18 (H-2' and H-6') to C-1 (δC 36.5), C-1' (δC 140.2), C-3' (δC 151.3), C-4' (δC 134.7) and C-6' and C-2' (δC 108.7), as well as the correlation from one OCH3 group at δH 3.75 to C-4' (δC 134.7). The proton substitutions on the B ring were assigned based on the 3J HMBC correlations between H-3 (δH 2.52) and C-6" (δC 120.5), and between H-5" (δH 6.38) and C-1" (δC 123.4). Moreover, the H-5" proton showed HMBC correlations to C-6" (δC 120.5), C-4" (δC 147.8) and C-3" (δC 134.9). The location of the remaining OCH3 group was at C-4", as deduced from the HMBC correlation between the signal at δH 3.78 and that of C-4". On the basis of the molecular formula of 1, the remaining four OH groups were located at C-2" (δC 144.7), C-3" (δC 134.9), C-3' (δC 151.3), and C-5' (δC 151.3). Bussealin A is thus assigned the structure 3',5',2",3"-tetrahydroxy-4',4"-dimethoxy-1,3-diphenylpropane (1).
Table 1.
1H and 13C NMR data for Bussealin A–D (1–4)a
| position | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| 1H (J, Hz) | 13C | 1H (J, Hz) | 13C | 1H (J, Hz) | 13C | 1H (J, Hz) | 13C | |
| 1 | 2.41 t (7.9) | 36.5 | 2.50 t (7.8) | 30.4 | 2.49 t (7.9) | 36.1 | 2.55 t (7.7) | 30.4 |
| 2 | 1.79 m | 33.0 | 1.76 m | 31.9 | 1.81 m | 33.2 | 1.79 m | 31.8 |
| 3 | 2.52 t (7.7) | 30.6 | 2.54 t (7.8) | 30.7 | 2.54 t (7.7) | 30.6 | 2.55 t (7.7) | 30.6 |
| 1' | 140.2 | 124.7 | 137.2 | 124.3 | ||||
| 2' | 6.18 s | 108.7 | 152.2 | 6.64 d (2.0) | 116.5 | 153.3 | ||
| 3' | 151.3 | 6.58 s | 99.4 | 147.3 | 6. 61 s | 99.6 | ||
| 4' | 134.7 | 147.2 | 147.0 | 149.1 | ||||
| 5' | 151.3 | 141.0 | 6.79 d (8.2) | 112.9 | 144.1 | |||
| 6' | 6.18 s | 108.7 | 6.60 s | 117.9 | 6.60 dd (8.2, 2.0) | 120.6 | 6.75 s | 116.3 |
| 1" | 123.4 | 123.7 | 123.5 | 123.6 | ||||
| 2" | 144.7 | 144.7 | 144.7 | 144.7 | ||||
| 3" | 134.9 | 134.9 | 135.0 | 134.9 | ||||
| 4" | 147.8 | 147.8 | 147.8 | 147.8 | ||||
| 5" | 6.38 d (8.4) | 103.8 | 6.39 d (8.4) | 103.8 | 6.39 d (8.4) | 103.9 | 6.39 d (8.4) | 103.9 |
| 6" | 6.50 d (8.4) | 120.5 | 6.51 d (8.4) | 120.4 | 6.50 d (8.5) | 120.5 | 6.51 d (8.3) | 120.4 |
| 2'-OMe | 3.76 s | 56.8 | 3.78 s | 56.6 | ||||
| 4'-OMe | 3.75 s | 60.8 | 3.83 s | 57.0 | 3.80 s | 56.6 | 3.82 s | 56.8 |
| 5'-OMe | 3.76 s | 57.6 | ||||||
| 4"-OMe | 3.78 s | 56.5 | 3.80 s | 56.6 | 3.80 s | 56.6 | 3.80 s | 56.9 |
In CD3OD; δ (ppm) 500 MHz for 1H and 125 MHz for 13C; multiplicities; J values (Hz) in parentheses.
Bussealin B (2) was obtained as an off-white amorphous solid. Its positive ESI-MS revealed a pseudomolecular ion peak at m/z 335.1512 [M + H]+ corresponding to molecular formula C18H23O6. The 1H NMR spectrum (Table 1) showed two singlets of an AX system at δH 6.58 (s) and 6.60 (s), two aromatic doublets of an AB system at δH 6.51 (d, J = 8.4) and 6.39 (d, J = 8.4), three OCH3 groups [δH 3.76 (s), 3.80 (s) and 3.83 (s)], and one multiplet and two triplet methylene groups at δH 1.76 and 2.54 (t, J = 7.8) and 2.50 (t, J = 7.8). Inspection of the 1H and 13C NMR spectra of 2 revealed close similarities with those of 1, except for the presence of an additional OCH3 signal and the chemical shifts of the AX system of ring A. The fact that the chemical shifts of the carbons of ring B of compounds 1 and 2 were superimposable (Table 1) indicated the presence of a 2",3"-dihydroxy-4"-methoxyphenyl group in 2. Interpretation of HMBC and NOESY experiments allowed us to determine the location of the OCH3 groups to be at 2', 4', and 4". The two singlet aromatic protons on ring A were assigned according to the observation of 3J HMBC correlations from H-6' (δH 6.60) to C-1 (δC 30.4) and from H-3' (δH 6.58) to C-1' (δC 124.7). Moreover, the proton signal of H-1 (δH 2.50) showed HMBC correlations with C-1' (δC 124.7), C-6' (δC 117.9) and the methoxylated carbon at C-2' (δC 152.2). This indicated that the third OCH3 group must be at C-4' or C-5'. NOESY correlations from H-3' (δH 6.58) to 2'-OMe (δH 3.76) and to 4'-OMe (δH 3.83) established the location of the methoxy group at C-4' and the hydroxy group at C-5'. The structure of bussealin B was thus assigned as 5',2",3"-trihydroxy-2',4',4"-trimethoxy-1,3-diphenylpropane.
Bussealin C (3) was obtained as an off-white amorphous solid. Its positive ESI-MS revealed a pseudomolecular ion peak at m/z 305.1384 [M + H]+ corresponding to molecular formula C17H21O5. Its 1H NMR and 13C NMR spectra (Table 1) indicated that 3 is also a diphenylpropane with a 2",3"-dihydroxy-4"-methoxyphenyl group substituted at C-3. The 1,3,4-trisubstituted A ring was determined by the proton coupling constants and HMBC correlations from H-2' (δH 6.64) and H-6' (δH 6.60) to C-1 (δC 36.1), and COSY correlations between H-5' (δH 6.79) and H-6' (δH 6.60). Furthermore, the HMBC spectrum showed a 3J correlation from H-6' to the methoxylated carbon at C-4' (δC 147.0), which was confirmed by NOESY correlations between H-5' (δH 6.79) and 4'-OMe (δH 3.80). The above data coupled with the molecular formula led to assignment of the structure of bussealin C as 3', 2",3"-trihydroxy-4',4"-dimethoxy-1,3-diphenylpropane.
Bussealin D (4) was obtained as an off-white amorphous solid. The positive ESI-MS exhibited a pseudomolecular ion peak at m/z 349.1648 [M + H]+ corresponding to the molecular formula C19H25O6. The 1H NMR and 13C NMR spectra (Table 1) indicated that 4 had the same tetrasubstituted B ring with an OCH3 group at C-4" as in compounds 1–3. In its 1H NMR spectrum, the coupling patterns and the locations of the aromatic proton resonances of ring A were very similar to those of 2. The presence of three OCH3 groups and the substitution pattern of ring A of compound 4 were deduced by interpretation of the 1D and 2D NMR data. The HMBC spectrum of 4 showed correlations from H-1 (δH 6.79) to C-1' (δC 124.3), C-6' (δC 116.3) and to the methoxylated carbon at C-2' (δC 153.3). Furthermore, a clear 3J long-range correlation from the singlet proton H-3' (δH 6.61) to C-1' (δC 124.3) was also observed. Thus, the two remaining OCH3 groups were determined to be at C-4' (δC 149.1) and C-5' (δC 144.1). The structure of bussealin D was thus determined to be 2",3"-dihydroxy-2',4',5',4"-tetramethoxy-1,3-diphenylpropane.
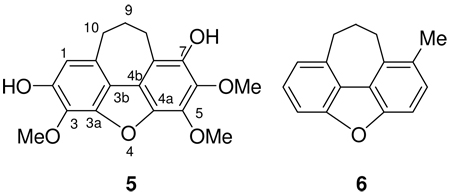
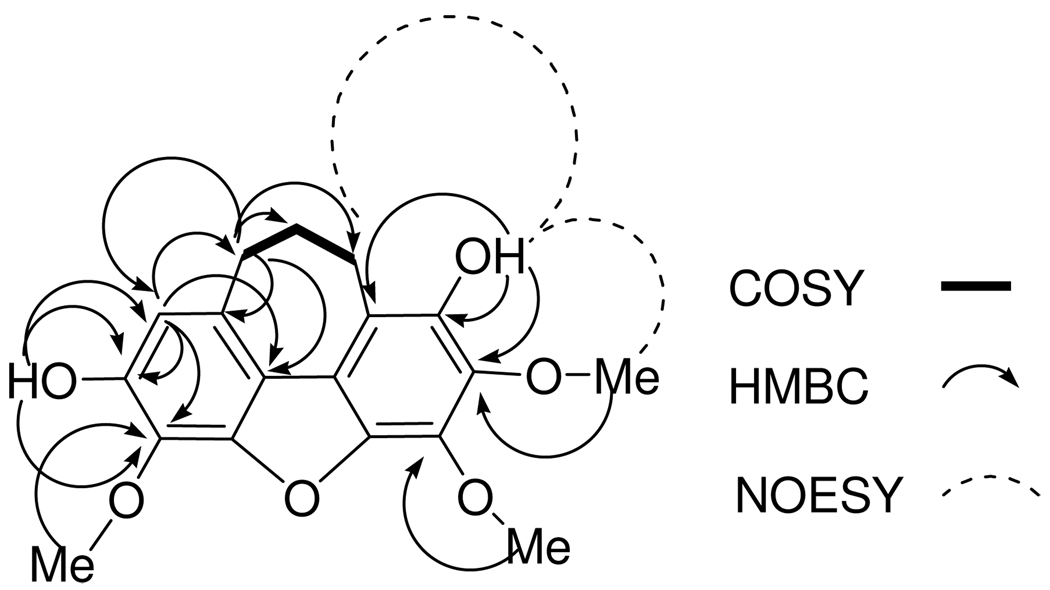
The positive ESI-MS of bussealin E (5) displayed a pseudomolecular ion peak at m/z 331.1181 [M + H]+ corresponding to the molecular formula C18H19O6. The 1H NMR spectrum in CDCl3 showed signals for a singlet aromatic proton at δH 6.70, two OH groups (δH 5.75 and 5.69), three OCH3 groups at δH 4.24, 4.24, and 4.01, and three methylene groups as multiplets at δH 3.13, 3.12 and 2.17. The 13C NMR spectrum of 5 exhibited 18 signals, assigned to three methylene (δC 35.5, 28.7, 24.3), three OCH3 (δC 60.8, 60.8 and 61.7), and twelve aromatic carbons of two isolated aromatic rings. Seven of the aromatic carbons were oxygenated, based on their deshielded chemical shifts (Table 2). The ten degrees of unsaturation implied by the molecular formula C18H18O6 required two additional rings. Interpretation of 1H-1H COSY, HMQC, HMBC and NOESY spectra allowed assignment of the locations of the functionalities present in 5. In the COSY spectrum, the three methylene groups were mutually coupled. The assignment of a singlet aromatic proton was substantiated by the observation of HMBC correlations from H-1 (δH 6.70) to C-10 (δC 35.5), C-3b (δC 118.2), and two oxygenated aromatic carbons at C-2 (δC 146.5) and C-3 (δC 129.7). HMBC correlations from the signal at δH 5.69 to C-1 (δC 110.1), C-2 (δC 146.5) and the methoxylated carbon at C-3 (δC 129.7) were observed, substantiating the location of a hydroxy group at C-2. The other hydroxy group was assigned to position 7 based on the observation of HMBC correlations from the signal at δH 5.75 to the carbon signals at C-6 (δC 136.5), C-7 (δC 142.3) and C-7a (δC 115.0). In addition, the signal at δH 5.75 showed NOESY correlations to H-8 (δH 3.13) and 6-OMe (δH 4.01). These observations required that the remaining OCH3 group be placed at C-5. Furthermore, the HMBC correlations observed from H-10 (δH 3.12) to C-1 (δC 110.1), C-10a (δC 131.7), C-3b (δC 118.2), C-8 (δC 28.7) and C-9 (δC 24.3) confirmed the location of the cycloheptadiene ring. The above data confirmed the cycloheptadibenzofuran skeleton of 5. Assignments of the 13C NMR signals of C-3a, C-4a and C-4b were made by comparing the measured data with those calculated by ACD/ChemSketch version 11.01. The calculated shifts were in excellent agreement with the observed values, and were all within the standard deviation of the software (5 ppm), except for C-7a. Therefore, the structure of 5 was assigned as 9,10-dihydro-2,7-dihydroxy-3,5,6-trimethoxy-8H-cyclohepta[klm]dibenzofuran.
Table 2.
1H and 13C NMR Data for Bussealin E (5)
| position | 1Ha | 13Ca | 13Cb | 1Hc |
|---|---|---|---|---|
| 1 | 6.70 s | 110.1 | 110.0 | 6.59 s |
| 2 | 146.5 | 149.1 | ||
| 3 | 129.7 | 131.3 | ||
| 3a | 146.2 | 148.7 | ||
| 3b | 118.2 | 113.3 | ||
| 4a | 140.7 | 139.7 | ||
| 4b | 120.6 | 117.4 | ||
| 5 | 135.6 | 137.0 | ||
| 6 | 136.5 | 137.9 | ||
| 7 | 142.3 | 145.1 | ||
| 7a | 115.0 | 109.7 | ||
| 8 | 3.13 m | 28.7 | 28.9 | 3.08 m |
| 9 | 2.17 m | 24.3 | 24.2 | 2.12 m |
| 10 | 3.12 m | 35.5 | 34.5 | 3.07 m |
| 10a | 131.7 | 128.6 | ||
| 3-OCH3 | 4.24 s | 60.8 | 61.5 | 4.18 s |
| 2-OH | 5.69 s | |||
| 5-OCH3 | 4.24 s | 60.8 | 61.6 | 4.08 s |
| 6-OCH3 | 4.01 s | 61.7 | 61.0 | 3.90 s |
| 7-OH | 5.75 s |
In CDCl3; δ (ppm) 600 MHz for 1H and 150 MHz for 13C; multiplicities; J values (Hz) in parentheses.
Calculated using ACD/ChemSketch version 11.01.
In CD3OD; δ (ppm) 600 MHz for 1H; multiplicities; J values (Hz) in parentheses.
It is noteworthy that bussealin E is the first cycloheptadibenzofuran isolated from natural sources, and the cycloheptadibenzofuran skeleton is rare among synthetic compounds. The only simple synthetic compound with this ring system is 9,10-dihydro-1-methyl-8H-cyclohepta[ klm]dibenzofuran (6) and its 8-keto derivative.5
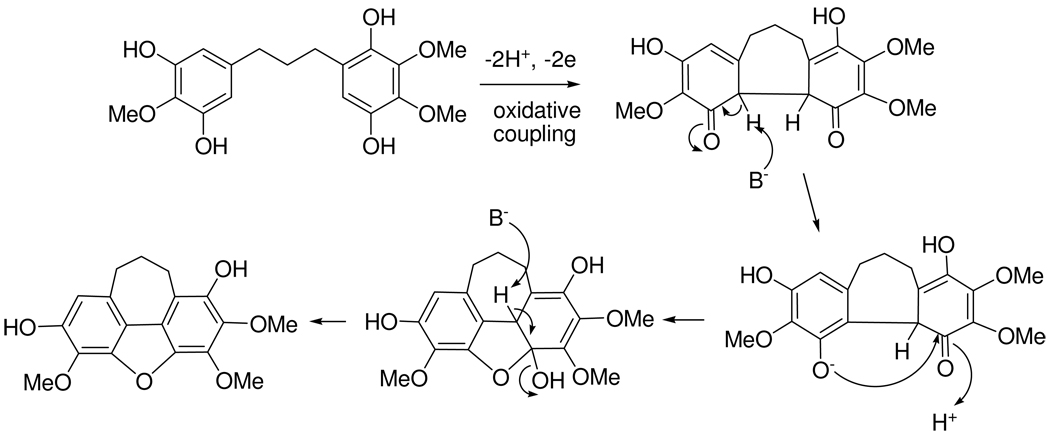
The presence of diphenylpropanes in B. sakalava suggests that bussealin E is biosynthesized by oxidative coupling of an appropriate precursor diphenylpropane. This could be followed by nucleophilic attack from a phenolate anion on a carbonyl group followed by dehydration to afford the new cycloheptadibenzofuran skeleton (5) as indicated in Scheme 1.
Scheme 1.
Possible biosynthesis of cycloheptadibenzofuran 5 in B. sakalava
The bioactivity of diphenylpropanes has been widely studied. The diphenylpropane broussonin A inhibited respiratory syncytial-virus (RSV) more effectively than the standard antiviral drug ribavirin,6 and its anti-aromatase activity has also been evaluated.7 Broussonin B moderately inhibited a chymotrypsin-like activity of the proteasome.8 The anti-inflammatory,9,10 antifungal,11 antivascular,12 adipogenic,13 and anti-hCNT3 (human concentrative nucleoside transporter 3)14 activities of diphenylpropane analogues have also been reported. Since there have been no previous studies on the properties of diphenylpropanes on human ovarian cancer cells, we investigated the antiproliferative activity of diphenylpropanes 1–4 against the A2780 human ovarian cancer cell line. Bussealins A–D (1–4) showed only weak antiproliferative activities, with IC50 values of 36, 24, 36, and 40 µM, respectively. Bussealin E (5), with a new chemical skeleton, was also tested against the A2780 cell line, but it also only exhibited weak activity with an IC50 value of 45 µM. The new skeleton of bussealin E thus does not appear to confer any novel antiproliferative activity beyond that which is normal for diphenylpropanes.
Experimental Section
General Experimental Procedures
UV and IR spectra were measured on a Shimadzu UV-1201 spectrophotometer and a MIDAC M-series FTIR spectrophotometer, respectively. NMR spectra were recorded in CD3OD or CDCl3 on either JEOL Eclipse 500 or Bruker Avance 600 spectrometers. The chemical shifts are given in δ (ppm) and coupling constants (J) are reported in Hz. Mass spectra were obtained on an Agilent 6220 LC-TOF-MS. HPLC was performed on a Shimadzu LC-10AT instrument with a semi-preparative C18 Varian Dynamax column (5 µm, 250 × 10 mm).
Antiproliferative Bioassays
Antiproliferative activities were obtained at Virginia Polytechnic Institute and State University against the drug-sensitive A2780 human ovarian cancer cell line as previously described, except that the samples were added in 1 µL 100% DMSO per well instead of 20 µL of 1:1 DMSO:H2O; paclitaxel (IC50 0.017 µM) was used as a positive control.15 The A2780 cell line is a drug-sensitive ovarian cancer cell line.16
Plant Material
A sample of root of Bussea sakalava Du Puy & R. Rabev. (Fabaceae) was collected on January 25, 2007, near Ambolobozobe, Madagascar at coordinates 12°31'26"S 49°31'29"E, at an elevation of 20 m. Its assigned collection number is Rakotonandrasana et al. 1079. The genus Bussea Harms is a small genus including 7 species (5 from Tropical Africa and 2 from Madagascar). B. sakalava is endemic to deciduous forest from western to northern Madagascar. The hard wood of this species is used in construction and as firewood.17 Voucher specimens have been deposited at the Parc Botanique and Zoologique de Tsimbazaza (TAN) and at the Centre National d'Application des Recherches Pharmaceutiques (CNARP) in Antananarivo, Madagascar; the Missouri Botanical Garden in St. Louis, Missouri (MO); and the Muséum National d'Histoire Naturelle in Paris, France (P).
Extraction and Isolation
Dried roots of B. sakalava (275 g) were ground in a hammer mill, then extracted with ethanol by percolation for 24 hours at room temperature to give the crude extract MG 4273 (14.4 g), of which 3.0 g was shipped to Virginia Polytechnic Institute and State University (VPISU) for bioassay-guided isolation. Sample MG 4273 (IC50 9.6 µg/mL, 2.1 g) was suspended in aqueous MeOH (MeOH-H2O, 9:1, 100 mL) and extracted with hexane (3 × 100 mL portions). The aqueous layer was then diluted to 60% MeOH (v/v) with H2O and extracted with CH2Cl2 (3 × 150 mL portions). The hexane extract was evaporated in vacuo to leave 227 mg with an IC50 value of 19 µg/mL. 102.9 mg of residue from the CH2Cl2 extract had an IC50 of 10 µg/mL. The aqueous MeOH extract (1.7 g) was inactive. The CH2Cl2 extract was selected for fractionation using an SPE cartridge over C-18, and two fractions were collected. Fractions I and II (70.2 mg and 26.8 mg) had IC50 values of 8.6 and 15 µg/mL, respectively. Fraction I was separated by C-18 HPLC (65% MeOH-H2O), and compounds 1 (3.3 mg tR 12.5 min), 2 (1.7 mg tR 18.6 min), 3 (2.0 mg tR 22.0 min), 4 (1.1 mg tR 29.5 min) and 5 (1.1 mg tR 26.5 min) were isolated.
3',5',2",3"-Tetrahydroxy-4',4"-dimethoxy-1,3-diphenyl-propane (1)
off-white amorphous solid; UV (MeOH) λmax nm (log ε) 218 (4.40), 267 (3.69), 294 (3.52); IR νmax cm−1: 3367, 1648, 1450, 1115, 1024. 1H NMR (500 MHz, CD3OD) and 13C NMR (125 MHz, CD3OD), see Table 1; ESI-MS m/z 321.1338 [M + H]+ (calcd for C17H21O6, 321.1338).
5',2",3"-Trihydroxy-2",4',4"-trimethoxy-1,3-diphenyl-propane (2)
off-white amorphous solid; UV (MeOH) λmax nm (log ε) 214 (4.25), 229 (sh) (4.10), 290 (3.59) nm; IR νmax cm−1: 3332, 1599, 1444, 1095, 1032; 1H NMR (500 MHz, CD3OD) and 13C NMR (125 MHz, CD3OD), see Table 1; ESI-MS m/z 335.1512 [M + H]+ (calcd for C18H23O6, 335.1495).
3',2",3"-Trihydroxy-4',4"-dimethoxy-1,3-diphenyl-propane (3)
off-white amorphous solid; UV (MeOH) λmax nm (log ε) 208 (4.15), 267 (3.54), 289 (3.47) nm; IR νmax cm−1: 3338, 1656, 1450, 1115, 1024; 1H NMR (500 MHz, CD3OD) and 13C NMR (125 MHz, CD3OD), see Table 1; ESI-MS m/z 305.1384 [M + H]+ (calcd for C17H21O5, 305.1389).
3',2",3"-Trihydroxy-4',4"-dimethoxy-1,3-diphenyl-propane (4)
off-white amorphous solid; UV (MeOH) λmax nm (log ε) 210 (4.21), 229 (sh) (4.01), 289 (3.48) nm; IR νmax cm−1: 3350, 1602, 1450, 1115, 1026; 1H NMR (500 MHz, CD3OD) and 13C NMR (125 MHz, CD3OD), see Table 1; ESI-MS m/z 349.1648 [M + H]+ (calcd for C19H25O6, 349.1651).
9,10-Dihydro-2,7-dihydroxy-3,5,6-trimethoxy-8H-cyclohepta[klm]dibenzofuran (5)
off-white amorphous solid; UV (MeOH) λmax nm (log ε) 218 (4.25), 270 (3.78), 294 (3.73), 316 (3.47)) nm; IR νmax cm−1: 3332, 1567, 1449, 1115, 1024; 1H NMR (600 MHz, CD3OD and CDCl3) and 13C NMR (150 MHz, CD3OD), see Table 2; ESI-MS m/z 331.1181 [M + H]+ (calcd for C18H19O6, 331.1182).
Supplementary Material
Figure 1.
COSY, HMBC and NOESY correlations of 5
Acknowledgments
This project was supported by the Fogarty International Center, the National Cancer Institute, the National Science Foundation, the National Heart, Lung and Blood Institute, the National Institute of Mental Health, the Office of Dietary Supplements, and the Office of the Director of NIH, under Cooperative Agreement U01 TW000313 with the International Cooperative Biodiversity Groups. This project was also supported by the Agricultural Food Research Initiative of the National Institute of Food and Agriculture, USDA, Grant #2008-35621-04732. These supports are gratefully acknowledged. This work was also supported by the National Science Foundation under Grant No CHE-0619382 for purchase of the Bruker Avance 600 NMR spectrometer and Grant No. CHE-0722638 for the purchase of the Agilent 6220 mass spectrometer. We thank Mr. B. Bebout and Dr. Mehdi Ashraf-Khorassani for obtaining the mass spectra and Dr. Hugo Azurmendi for assistance with the NMR spectra. Fieldwork essential for this project was conducted under a collaborative agreement between the Missouri Botanical Garden and the Parc Botanique et Zoologique de Tsimbazaza and a multilateral agreement between the ICBG partners, including the Centre National d’Applications des Recherches Pharmaceutiques. We gratefully acknowledge courtesies extended by the Government of Madagascar (Ministère des Eaux et Forêts).
Footnotes
Supporting Information Available: 1H, 13C, COSY, HMBC, HMQC, and NOESY spectra of bussealins A–E (1–5). This information is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Biodiversity Conservation and Drug Discovery in Madagascar, Part 44. For Part 43, see Cao S, Hou Y, Brodie P, Miller JS, Randrianaivo R, Rakotobe E, Rasamison VE, Kingston DGI. Chem. Biodiversity. 2010 doi: 10.1002/cbdv.201000061. doi: 10.1002/cbdv.201000061.
- 2.Evans CS, Bell EA. Phytochemistry. 1978;17:1127–1129. [Google Scholar]
- 3.Watson R, Fowden L. Phytochemistry. 1973;12:617–622. [Google Scholar]
- 4.Freiburghaus F, Kaminsky R, Nkunya MHH, Brun R. J. Ethnopharm. 1996;55:1–11. doi: 10.1016/s0378-8741(96)01463-8. [DOI] [PubMed] [Google Scholar]
- 5.Cagniant P, Bellinger N, Cagniant D. C. R. Acad. Sci., Série C. 1973;277:383–385. [Google Scholar]
- 6.Bae G, Yu JR, Lee J, Chang J, Seo EK. Chem. Biodivers. 2007;4:2231–2235. doi: 10.1002/cbdv.200790181. [DOI] [PubMed] [Google Scholar]
- 7.Lee D, Bhat KPL, Fong HHS, Farnsworth NR, Pezzuto JM, Kinghorn AD. J. Nat. Prod. 2001;64:1286–1293. doi: 10.1021/np010288l. [DOI] [PubMed] [Google Scholar]
- 8.Tsukamoto S, Wakana T, Koimaru K, Yoshida T, Sato M, Ohta T. Biol. Pharm. Bull. 2005;28:1798–1800. doi: 10.1248/bpb.28.1798. [DOI] [PubMed] [Google Scholar]
- 9.Leu YL, Hwang TL, Chung YM, Hong PY. Chem. Pharm. Bull. 2006;54:1063–1066. doi: 10.1248/cpb.54.1063. [DOI] [PubMed] [Google Scholar]
- 10.Sawle P, Moulton BE, Jarzykowska M, Green CJ, Foresti R, Fairlamb IJS, Motterlini R. Chem. Res. Toxicol. 2008;21:1484–1494. doi: 10.1021/tx800115g. [DOI] [PubMed] [Google Scholar]
- 11.Lopes NP, Kato MJ, Yoshida M. Phytochemistry. 1999;51:29–33. [Google Scholar]
- 12.Ducki S, Rennison D, Woo M, Kendall A, Chabert JFD, McGown AT, Lawrence NJ. Bioorg. Med. Chem. 2009;17:7698–7710. doi: 10.1016/j.bmc.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 13.Youn UJ, Lee YS, Jeong H, Lee J, Nam JW, Lee YJ, Hwang ES, Lee JH, Lee D, Kang SS, Seo EK. J. Nat. Prod. 2009;72:1895–1898. doi: 10.1021/np900397f. [DOI] [PubMed] [Google Scholar]
- 14.Gupte A, Buolamwini JK. Bioorg. Med. Chem. Lett. 2009;19:917–921. doi: 10.1016/j.bmcl.2008.11.112. [DOI] [PubMed] [Google Scholar]
- 15.Cao S, Brodie PJ, Miller JS, Randrianaivo R, Ratovoson F, Birkinshaw C, Andriantsiferana R, Rasamison VE, Kingston DGI. J. Nat. Prod. 2007;70:679–681. doi: 10.1021/np060627g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louie KG, Behrens BC, Kinsella TJ, Hamilton TC, Grotzinger KR, McKoy WM, Winker MA, Ozols RF. Cancer Res. 1985;45:2110–2115. [PubMed] [Google Scholar]
- 17.Du Puy DJ, Labat J-N, Rabevohitra R, Villiers J-F, Bosser J, Moat J. The Leguminosae of Madagascar. Kew: Royal Botanical Gardens; 2002. pp. 22–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.