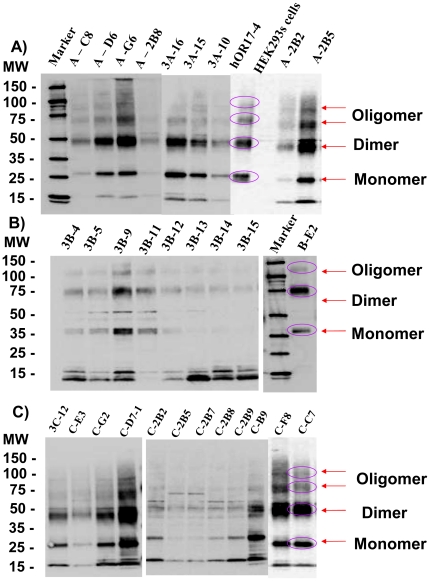
Figure 2. Western blot analyses of selected HEK293s cell clones expressing OR131-2 proteins after 48 hours treatment with 1 µg/mL tetracycline and 2.5 mM sodium butyrate.
Fos-Choline14 soluble proteins isolated from harvested cells, were separated on Novex® 4–12% Bis-Tris gels, blotted on Polyvinylidene Fluoride (PVDF) membranes and stained with Rho1D4 monoclonal antibody. A) HEK293s cell clones expressing OR131-2A with protein sequence shown in supplementary Figure 1. Purified Rho1D4-tagged human olfactory receptor 17-4 (hOR17-4) solubilized in Fos-Choline 14 was loaded in gel as positive controls while the same concentration of Fos-Choline dissolved cell lysates from un-transfected HEK293s cells served as negative control. Similar to purified hOR17-4, OR131-2 proteins migrated faster on SDS gels than what their theoretical molecular weights predict. Monomeric, dimeric and oligomeric forms of the proteins were visible on the gel. B) HEK293s cell clones expressing OR131-2B with protein sequence as shown in supplementary Figure 1. The insertion of T4-lysozyme in the third intracellular loop of OR131-2 results in an expected gel shift of the protein monomer, dimer and oligomers. C) HEK293s cell clones expressing OR131-2C with sequence shown in supplementary Figure S1.