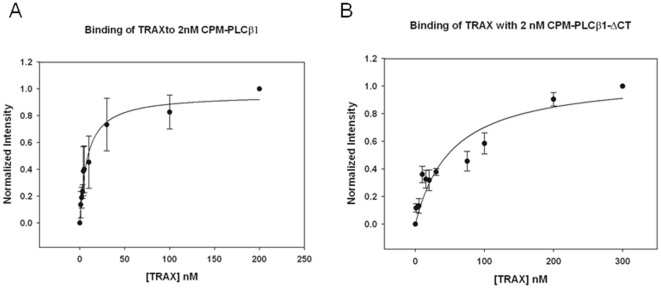
Figure 2. TRAX binds strongly to PLCβ1.
A – Binding of TRAX to 2 nM CPM- PLCβ1 as monitored by the increase in CPM intensity where the normalized fluorescence intensity is shown as a function of TRAX concentration. In these studies, an 80% increase in intensity was observed as compared to control samples that substituted buffer for TRAX. Also shown is the fitted curve to a bimolecular dissociation constant where Kd = 8±1 nM (n = 6 and S.D. is shown). B – Identical study as 2A except that the COOH-terminal deletion mutant of PLCβ1 (PLCβ1-ΔC) was used instead of the full length enzyme (n = 3 and S.D. is shown). While a binding curve is shown to guide the eye, the affinity between the proteins was too weak to be accurately fit to a bimolecular dissociation constant. We note that the total change in CPM intensity was also ∼80% at the end of the titration.