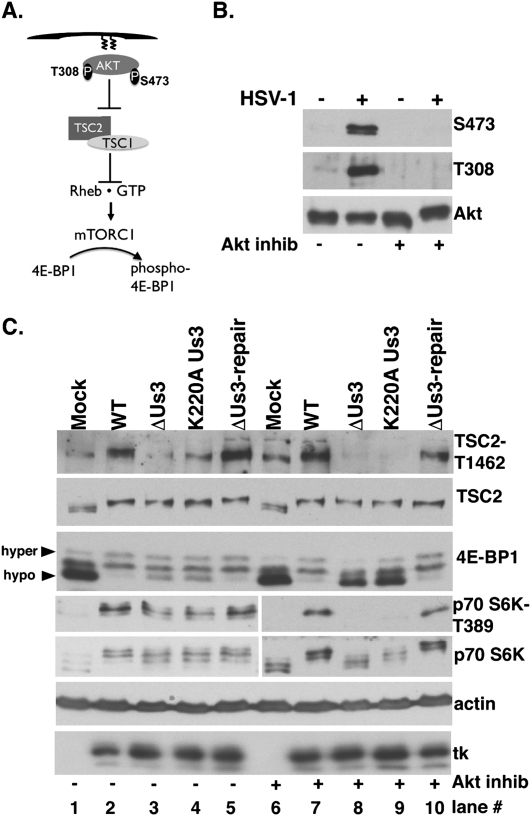
Figure 3.
Inhibition of Akt does not suppress Us3-dependent 4E-BP1 and TSC2 phosphorylation in HSV-1-infected cells. (A) Activation of mTORC1 by Akt. Once plasma membrane localized via a lipid-binding plekstrin homology domain, Akt is activated by T308/S473 phosphorylation. Akt inhibits TSC rheb-GAP activity by phosphorylating TSC2 on T1462/S939, promoting rheb•GTP-mediated mTORC1 activation and subsequent 4E-BP1 hyperphosphorylation. (B) Growth-arrested NHDFs were mock-infected (−) or infected (MOI = 5) with wild-type (WT) HSV-1 (+). After 4 h, cells were treated with DMSO or Akt inhibitor VIII (5 μM). At 9 hpi, total protein was fractionated by SDS-PAGE and analyzed by immunoblotting with indicated antibodies (S473, T308-phospho specific Akt antibodies, and Akt total). (C) As in B, except ΔUs3, K220A Us3, and Us3 repair were included. Total protein was isolated at 15 hpi, fractionated by SDS-PAGE, and analyzed by immunoblotting with the indicated antisera: TSC2-T1462 (phospho-specific T1462 antibody), TSC2 (total TSC2), anti-4E-BP1, anti-HSV-1 tk antibody, S6K-T389 (phospho-specific T389 p70 S6K), and total p70 S6K. Different phospho-4E-BP1 forms were resolved (hyper vs. hypo) as described in Figure 2A.