Fig. 2.
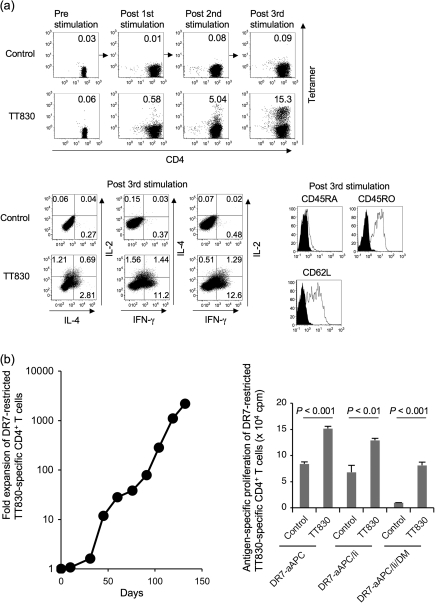
DR-aAPC/Ii/DM can generate long-lived antigen-specific CD4+ T cells. (a) DR1-aAPC/Ii/DM can expand DR1-restricted antigen-specific Th1-biased CD4+ T cells with a central memory phenotype in the presence of low dose IL-2 and IL-15. Purified DR1+ CD4+ T cells were stimulated every 10 days with TT830 peptide-pulsed and -irradiated DR1-aAPC/Ii/DM. Following each stimulation, IL-2 (10 IU ml−1) and IL-15 (10 ng ml−1) were added to the T-cell cultures every 3 days. The percentage of DR1-restricted TT830-specific CD4+ T cells was determined by TT830 tetramer staining (top). DR1-restricted CLIP-specific tetramer was employed as a negative control. Following three stimulations (day 28), IL-2, IL-4 and IFN-γ secretion was also analyzed by intracellular cytokine staining (bottom left). DR1-transduced T2 cells were pulsed with TT830 or pan-DR PADRE (control) peptide and were used for stimulation. Cells were co-stained with anti-CD45RA, CD45RO and CD62L mAbs along with TT830-tetramer (bottom right). Expression on gated tetramer-staining cells is depicted. Isotype staining was used as a control. (b) DR-aAPC/Ii/DM was able to generate long-lived antigen-specific CD4+ T cells. Purified CD4+ T cells isolated from DR7-positive healthy donors were repeatedly stimulated with TT830-pulsed and -irradiated DR7-aAPC/Ii/DM every 14 days. Between stimulations, 10 IU ml−1 IL-2 and 10 ng ml−1 IL-15 were added to the T-cell cultures. To confirm the antigen specificity of the long-lived DR7-restricted TT830-specific T cells, antigen-specific proliferation was measured on day 103 using pan-DR PADRE (control) or TT830 peptide-pulsed and mitomycin-C treated DR7-aAPC, DR7-aAPC/Ii or DR7-aAPC/II/DM as a stimulator. Data shown represents means ± SDs of triplicates. A TT830-specific proliferation assay was also performed on days 77, 91 and 110 with similar results (data not shown). In addition, TT830 specificity was confirmed by IFN-γ ELISPOT on day 150 (data not shown).