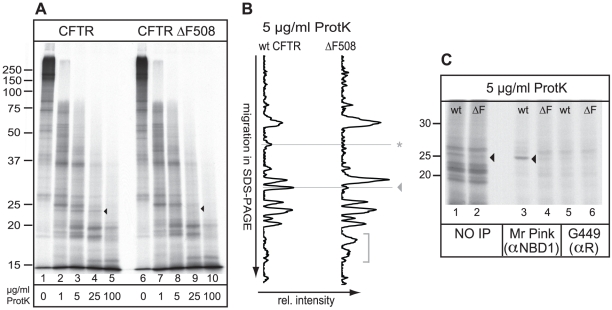
Figure 1. Minimal and local misfolding of ΔF508 CFTR.
(A) Both CFTR and ΔF508 CFTR were translated in vitro in the presence of 35S-methionine and cysteine and semi-permeabilized HT1080 cells for 60 min. Cells containing radiolabeled CFTR proteins were washed, lysed in Triton X-100, and prepared for limited proteolysis using increasing concentrations of proteinase K. The proteolytic digests were analyzed by 12% SDS-PAGE. The conformational difference between wild-type CFTR and ΔF508 CFTR is indicated by an arrowhead. (B) Relative intensities of all protease resistant fragments from a total 5 µg/ml Proteinase K digest, as in Figure 1A, were determined by total lane quantitation (Quantity One software Biorad). The y-axis represents electrophoretic mobility in 12% SDS-PA gel and the x-axis the relative intensity of the protease resistant fragments. The horizontal lines indicate the structural differences as described in A. The horizontal line indicated with an asterisk represents yet unidentified changes in the proteolytic pattern as a result of the ΔF508 mutation. The bracket represents small proteolytic fractions detected in both mutants. (C) Wild-type and ΔF508 CFTR were synthesized as in a, were subjected to 5 µg/ml proteinase K and NBD1-originated fragments were immunoprecipitated with polyclonal antibodies directed against NBD1 (Mr Pink) or against the R-region (G449). Arrowhead marks the NBD1-related fragment.