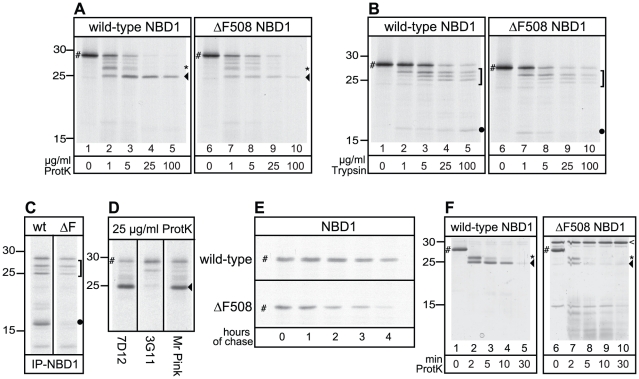
Figure 2. The effect of ΔF508 mutation on NBD1 alone.
(A) Wild-type and ΔF508 NBD1 were in vitro translated for 30 min, treated with indicated proteinase K concentrations as in Figure 1, and analyzed using 15% SDS-PAGE. The full length NBD1 domain is indicated by “#”, the asterisk (*) indicates the 27 kDa fragment, arrowhead (◂) indicates the 25 kDa fragment. (B) Similar experimental conditions as described in A, but using TPCK-trypsin as protease. A bracket (]) marks the triplet of protease resistant fragments and the dot (•) marks the 17 kDa fragment. (C) Wild-type and ΔF508 NBD1 were synthesized as in A, treated with 100 µg/ml trypsin, and fragments were immunoprecipitated with antibody 7D12 against NBD1. Fragments are labeled similar as in B. (D) Wild-type NBD1 was synthesized as in A, treated with 25 µg/ml proteinase K, and fragments were immunoprecipitated with the 7D12, 3G11 and Mr Pink antibody, recognizing specific epitopes within NBD1. Fragments are labeled similar as in A. (E) CHO cells expressing wild-type or ΔF508 NBD1 were pulse-labeled with 35S-methionine and cysteine for 5 min and chased for indicated times. NBD1 was immunoprecipitated using polyclonal antibody Mr Pink and analyzed using 15% SDS-PAGE. NBD1 indicated by “#”. (F) Purified human wild-type and ΔF508 NBD1, indicated by “#”, were incubated with 2 µg/ml Proteinase K for 0, 2, 5, 10 and 30 minutes at room temperature. Proteolytic digests were separated using 15% SDS-PAGE and visualized by silver staining. Asterisk (*) and arrowhead (◂) indicate 27 and 25 kDa fragments resp., and are similar as in A. The open arrowhead (<) indicates a protease resistant background band in the purified ΔF508 NBD1.