Fig 6.
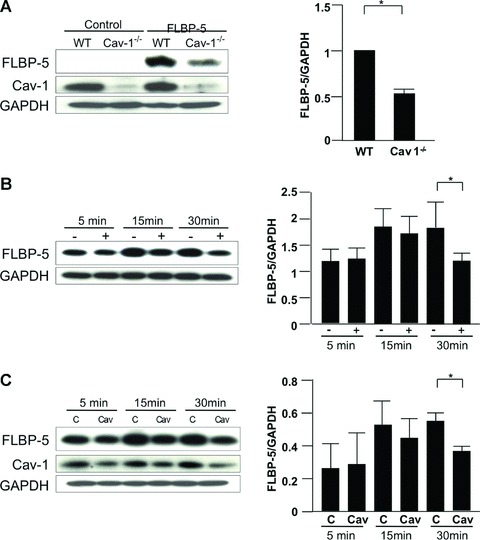
Internalization of IGFBP-5. (A) WT and Cav-1−/− fibroblasts were plated at 70% confluence in serum-free media. FLAG-tagged IGFBP-5 (FLBP-5) was added to each well. After 15 min., lysates were harvested and subjected to Western blot analysis. IGFBP-5 in lysates from WT and Cav-1−/− fibroblasts was detected using anti-FLAG M2 antibody. GAPDH was used as a loading control. Normalized FLBP-5 levels in WT fibroblasts were arbitrarily set at 1. Horizontal bars indicate mean values of two independent experiments. The unpaired t-test was used for statistical analysis. *P < 0.05. (B) WT fibroblasts were cultured with (+) or without (-) 10 mM MβCD for 1 hr in serum-free media, and FLBP-5 was added to each well. After 5, 15 and 30 min., lysates were harvested and subjected to Western blot analysis. IGFBP-5 in lysates was detected using anti-FLAG M2 antibody. GAPDH was used as a loading control. Horizontal bars indicate mean values of three independent experiments. Data were analysed using the paired t-test. *P < 0.05. (C) WT fibroblasts were transfected with control scrambled siRNA (C) or siCav-1 (Cav) for 48 hrs, and then FLBP-5 was added to each well. After 5, 15 and 30 min., lysates were harvested and subjected to Western blot analysis. IGFBP-5 in lysates was detected using anti-FLAG M2 antibody. GAPDH was used as a loading control. Horizontal bars indicate mean values of three independent experiments. Data were analysed using the paired t-test. *P < 0.05.
