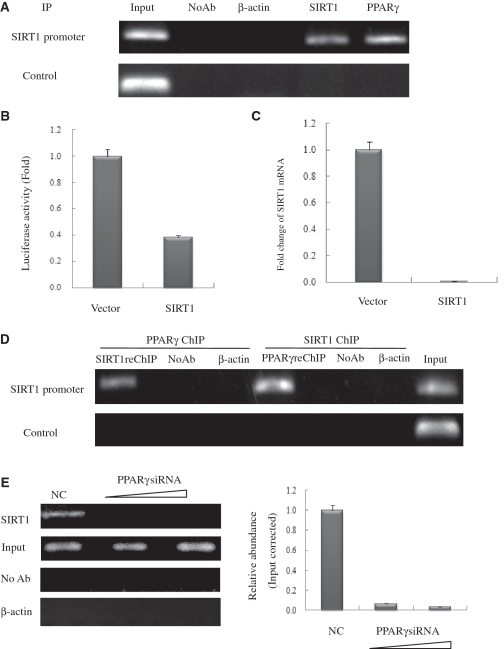
Figure 4.
PPARγ interacts with the endogenous SIRT1 promoter and recruits SIRT1 in HeLa cells. (A) Soluble chromatin was prepared from HeLa cells for ChIP analysis of the SIRT1 promoter using antibodies indicated. Precipitated DNA samples were amplified by PCR using a pair of primers that amplify the –592 bp to –163 bp region with GAPDH as an unrelated primers control. (B) Luciferase reporter assay of the SIRT1 promoter in SIRT1 transfected HeLa cells. HeLa cells were transfected with expression plasmids vector and SIRT1 as shown, together with SIRT1–Luc. Luciferase activities were measured 48 h after treatment. Values are the mean ± SD of triplicate data points from a representative experiment (n = 3), which was repeated three times with similar results. (C) Real-time quantitative PCR analysis of endogenous SIRT1 mRNA levels in SIRT1-transfected HeLa cells. HeLa cells were transfected with 10 µg of vector or pCDNA3.1-SIRT1. GAPDH transcript was used as a internal control. The error bar represents 1 SD. (D) ChIP-upon-ChIP assay showing the colocalization of PPARγ with SIRT1 at the SIRT1 promoter. Soluble chromatin was first immunoprecipitated with rabbit SIRT1 antibody, and the eluted product was re-immunoprecipitated with rabbit PPARγ antibody. ChIP-upon-ChIP assay was also performed with immunoprecipitation in reverse order, that is, PPARγ ChIP followed by SIRT1ChIP. (E) PPARγ recruites endogenous SIRT1 to its promoter in PPARγ knockdown cells in a dose-dependent manner. Real-time quantitative PCR verified the occurrence of recruitment. HeLa cells transfected with 10- or 20-nM PPARγ siRNA (triangle) were processed for ChIP uing SIRT1 antibody. The PCR primers amplified the SIRT1 promoter region as indicated in (A). For negative controls, a sample that did not contain antibody (No Ab) was immunoprecipitated, and antibody against β-actin was used as an unrelated antibody control.