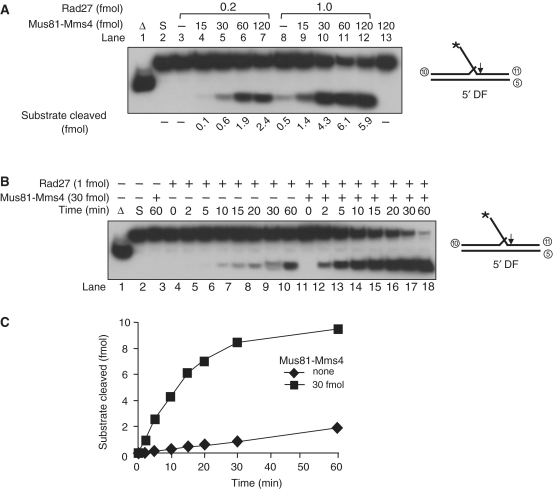
Figure 2.
The Mus81–Mms4 complex stimulates the endonuclease activity of Rad27. (A) Standard reactions for Rad27 were carried out with two different levels (0.2 or 1 fmol) of Rad27 in the presence of increasing amounts (15, 30, 60 and 120 fmol) of Mus81–Mms4. Reaction mixtures contained 25 mM Tris–HCl/pH 8.0, 2 mM MgCl2, 2 mM DTT, 0.25 mg/ml BSA and 10 fmol of the 5′DF substrate. The schematic structure of the substrate is as shown, and oligonucleotides used to construct the substrate are indicated by encircled numbers (Table 1). The reaction mixtures, after addition of indicating enzyme levels, were incubated for 15 min at 37°C. Reactions were terminated with 0.2% SDS, 10 µg proteinase K, followed by an additional incubation for 15 min at 37°C. The reaction products were analyzed similarly as described in Figure 1C. The arrows on the substrates indicate sites of cleavage. Asterisk indicates the position of 32P-labels at 5′ DNA ends. Open triangle indicates boiled substrate control. S, substrate only control. (B) A time-course experiment of Rad27 endonuclease activity in the absence (–) or presence (+, 30 fmol) of Mus81–Mms4. The reaction mixtures containing the 10 fmol of 5′DF substrates were added with 1 fmol of Rad27, and the mixtures were incubated for varying periods as indicated. The cleavage products were analyzed in a native 10% polyacrylamide gel. (C) The amount (fmol) of cleavage products formed in (B) was plotted against incubation time.