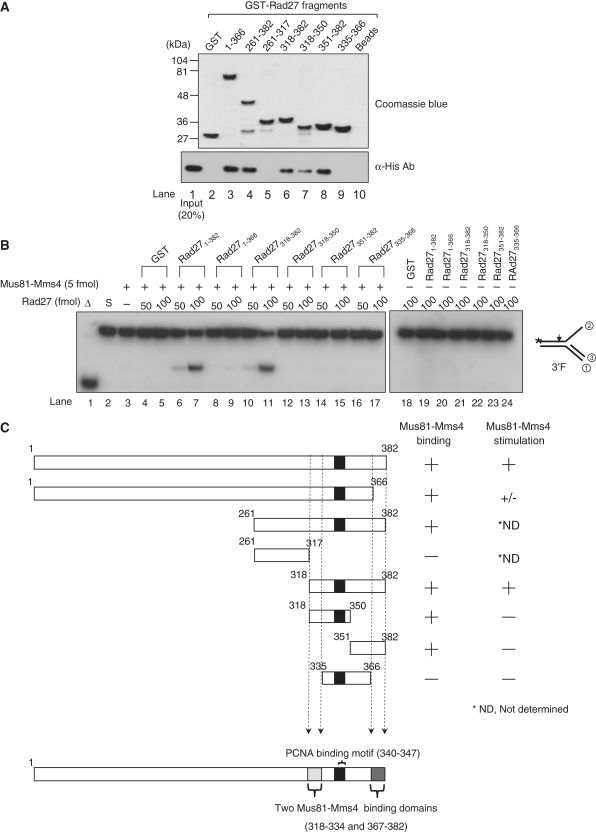
Figure 6.
Mapping of specific Rad27 domains required for binding and stimulation of Mus81–Mms4 activity. (A) The numbers above the gel indicate amino acid positions present in the GST-fusion Rad27 derivatives. GST indicates glutathione-S transferase. Beads, Mus81–Mms4 incubated with glutathione-agarose beads in the absence of Rad27. GST-fusion Rad27 fragments bound to glutathione-agarose beads were analyzed in a 10% SDS-polyacrylamide gel and stained with Coomassie blue (top gel, Commassie blue). For GST-pull-down assays (bottom gel), 1 pmol of Mus81–Mms4 was mixed with 100 pmol of GST (lane 2) or GST-fused Rad27 fragments (lanes 2–9) in binding buffer (25 mM HEPES-NaOH/pH7.5, 150 mM NaCl, 10% glycerol, and 0.01% NP40). The reaction mixtures were incubated for 1 h at 4°C with rocking. The complex formed was collected by centrifugation and washed. The presence of Mus81–Mms4 in the precipitated materials was determined by western blotting using anti-His monoclonal antibodies against His6-Mms4 (α-His Ab, bottom panel). Lane 1 contained 0.2 pmol of Mus81–Mms4 as an input control. (B) The reaction mixtures contained a fixed amount (5 fmol) of Mus81–Mms4, and the reactions were carried out with 10 fmol of the 3′F substrate and two levels (50 or 100 fmol) of GST-fused Rad27 fragments using standard reaction condition as described in ‘Materials and Methods’ section. The cleavage products formed from the 3′ F substrates were analyzed as described in Figure 2B. (C) A schematic summary of the results obtained in (A) and (B). The numbers in each fragment indicate the positions of amino acids present in full-length Rad27.