Figure 5.
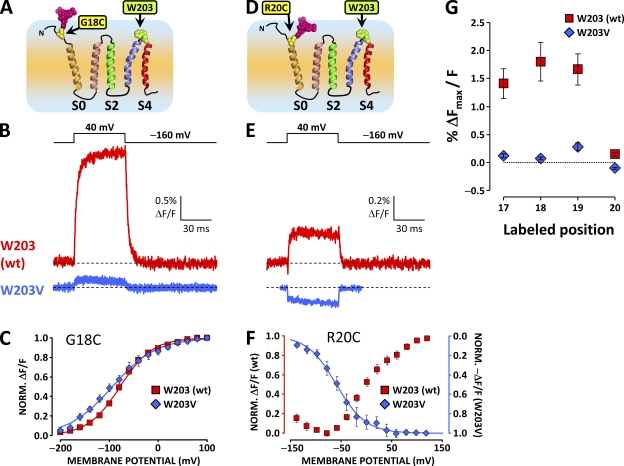
A large component of the ΔF reported from the S0 extracellular flank is abolished by the substitution of W203 at the extracellular tip of S4. (A) Putative topology of the BKCa channel α subunit voltage sensor domain. A unique cysteine was substituted at position 18 at the putative N-terminal flank of S0 to resolve conformational rearrangements from this region. W203 is also indicated in green. (B) TMRM fluorescence traces recorded during depolarization to 40 mV from wt channels labeled at position 18 (red trace, as in Fig. 2 G) and channels bearing mutation W203V (blue trace). Note the dramatically diminished ΔF/F signal amplitude. (C) Normalized TMRM fluorescence from channels with mutation W203V (blue diamonds) and without (red squares), plotted against membrane potential and fitted with Boltzmann distributions (blue and red curves, respectively). Boltzmann parameters are listed in Table I. Error bars represent SEM. (D–F) As in A–C, respectively, for wt and W203V channels labeled at position 20. In this position, the fluorophore is apparently influenced by two voltage-dependent processes with opposite effects on its fluorescence intensity (Fig. 4). Mutation W203V diminishes the voltage-dependent unquenching process, but it does not apparently affect the residual voltage-dependent quenching process (E). (G) Mean fitted ΔFmax/F signal, normalized for fitted maximum conductance to normalize for channel expression, plotted against labeled position, for wt and W203V channels. Mutation W203V diminishes the ΔF/F observed from positions 17–19 by ≈90%. At position 20, mutation W203V removes the unquenching process (positive ΔF/F) to reveal a residual negative ΔF/F signal. Error bars represent SEM.